Understanding how a contact lens performs on eye, and how
the material characteristics of a lens affect performance is key
to maintaining healthy eyes and happy patients. Silicone hydrogels
have many of the positive attributes of soft contact lenses such
as good initial comfort, and the movement and wetting characteristics
of conventional hydrogels. However practitioners should be aware
of the differences between these lens types and not assume that all
silicone hydrogels will behave in the same way as conventional hydrogels.
In 1985, the United States Food and Drug Administration (FDA) devised four contact lens groups as part of its draft guidelines for testing contact lens care products (Table 1). The groupings classify lenses by ionicity and water content, and when they were first introduced enabled practitioners to predict contact lens performance on eye and make informed decisions on the compatibility between lenses and the overwhelming number of lens care products that were then available (1). Since then the general assumption has been that contact lenses within the same FDA group will behave similarly and that contact lens solutions approved for use with an FDA group will be suitable for all lenses classified within that group.
All hydrogels are formed by the cross-linking of chains of monomeric units into a matrix-like polymer, and the unique attributes of each polymer are defined by the interaction of chemical groups and degree of cross-linking. Silicone hydrogels share a similar structure with conventional hydrogels and therefore have been classified into the FDA Groups 1 and III (Table 1). However silicone hydrogels differ markedly in chemical composition from conventional hydrogels and their FDA classification gives practitioners no indication of the unique characteristics that incorporation of silicone and other monomers impart to lens performance.
Silicone hydrogels vs hydrogels
The most striking difference between conventional and silicone hydrogels is that unlike conventional hydrogels, the high oxygen transmissibility of silicone hydrogels is not dependent on the water content of the lens material.
Conventional hydrogels are primarily composed of hydrophilic monomers that attract and bind water into the polymer. Oxygen dissolved in the water is transported through the lens to the cornea and the higher the water content, the higher the oxygen permeability of the material. The main component of conventional hydrogels is polyHEMA (38% water content) and other monomers are added to improve wettability and oxygen transport. Water content can be increased by the addition of methacrylic acid or by incorporating a higher proportion of hydrophilic neutral groups such as polyvinyl alcohol (PVA) or N-vinyl pyrrolidone (NVP). For more detail see Lyndon Jones and Brian Tighe’s previous editorial Silicone Hydrogel Contact Lens Materials Update - Part 1.
In contrast, the high oxygen transmissibility of silicone hydrogels is a direct consequence of the addition of siloxanes into the polymer. Increasing the proportion of siloxanes into the polymer, increases oxygen permeability but also lowers the proportion of hydrophilic monomers that can be incorporated, which in turn leads to a reduction in water content. In addition, siloxane groups are hydrophobic in nature which means silicone hydrogels need to be surface treated to increase wettability and to reduce the level of lipid deposits that would occur if the surface was left untreated. For more detail see Lyndon Jones and Brian Tighe’s previous editorial Silicone Hydrogel Contact Lens Materials Update - Part 2.
Compatibility with lens care solutions
Although allowed for up to 30 nights of continuous wear, silicone hydrogels are being used as a first choice lens for patients on a daily wear basis in increasing numbers (Figure 1). Based on the millions of conventional lens wearers who successfully use multipurpose solutions, practitioners overwhelmingly recommend multipurpose solutions for silicone hydrogel lens maintenance and disinfection. However there is increasing evidence that a significant number of silicone hydrogel wearers develop unacceptable levels of staining with some solutions.
Overall studies comparing the effects of different lens care and disinfection systems with silicone hydrogels indicate that little or no solution-based staining occurs with hydrogen peroxide based or polyquaternium-1 based systems (2-4). However several anecdotal reports and clinical studies indicate that different silicone hydrogel lenses vary in their interaction with polyaminopropyl biguanide (PHMB)-based multipurpose solutions (2,3,5-7). In addition, Amos has found that the same silicone hydrogel will interact differently with multipurpose solutions with the same active ingredient (PHMB) but of different formulation (8). 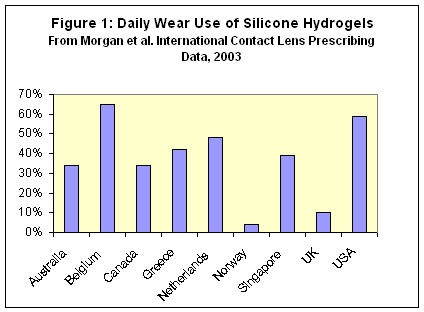
Discussion The recent launch of two new silicone hydrogels for daily wear and flexiwear has provided practitioners with a broader range of options for their patients and will drive the need for lens care solutions that are compatible with silicone hydrogels. Practitioners should keep in mind that any unusual signs and symptoms that occur after transferring patients from conventional hydrogels to silicone hydrogels (or from one silicone hydrogel to another) without changing lens care system, may be caused by the combination of lens type with care solution rather than by the change in lens type.
The vast differences between silicone hydrogels and conventional
hydrogels in chemical composition and on-eye behaviour suggest
that perhaps a new FDA lens group should be instituted that better
describes the unique characteristics of silicone hydrogels.
References
1. Stone R. Why contact lens groups? Contact Lens Spectrum. 1988;Dec:38-41.
2. Jones L, MacDougall N and Sorbara L. Asymptomatic corneal staining associated with the use of balafilcon silicone-hydrogel contact lenses disinfected with a polyaminopropyl biguanide-preserved care regimen. Optom Vis Sci. 2002;79:753-761.
3. Jones L. Understanding incompatibilities. Contact Lens Spectrum. 2004;18:4-7.
4. Amos C. A clinical comparison of two soft lens care systems used with silicone hydrogel contact lenses. Optician. 2004;227:16-20.
5. Epstein A. SPK with daily wear of silicone hydrogel lenses and MPS. Contact Lens Spectrum. 2002;17:30.
6. Fonn D. Observations of corneal staining with MPS and silicone hydrogel lenses. Contact Lens Spectrum. 2002;17:32.
7. Sentell K and Beaullieu E. Comparison of preservative uptake and release profiles of PHMB from soft conact lens care products by silicone hydrogel contact lenses. In: ARVO Annual Meeting, Fort Lauderdale, Florida, 2004.
8. Amos C. Performance of a new multipurpose solution used with silicone hydrogels. Optician. 2004;227:18-22.
|