Continued
< Part 1 Part
2:
This, the second part of a two-part review of silicone hydrogel
materials, discusses the differences between conventional and
silicone hydrogel lenses in terms of their surface and bulk properties.
1. Bulk Properties Oxygen Transmissibility
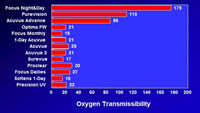 |
| Figure 1 - click to enlarge |
In silicone hydrogel materials the oxygen is primarily transmitted
through the silicone component of the lens material, resulting
in a dramatic increase in the oxygen permeability of the materials
(Figure 1).
Clinical studies have confirmed that many of the long and short-term
hypoxic problems seen with extended-wear of traditional lenses
are overcome with these novel, highly permeable materials.(1-8)
Mechanical Properties
The material elasticity of currently marketed silicone hydrogels
is much less than silicone elastomer lenses, but silicone hydrogel
lenses remain “stiffer” than conventional hydrogels,
due to the incorporation of silicone. The modulus of the first two
silicone hydrogel materials is some 4-6 times greater than low rigidity
materials such as etafilcon A. The modulus of Acuvue Advance is
much closer to conventional materials, being only 1.5 times more
rigid than etafilcon.(9) According to Johnson & Johnson, this
reduced stiffness is due to the reduced amount of silicone present,
along with benefits due to the internal wetting agent HydraClear™,
which is based upon polyvinyl
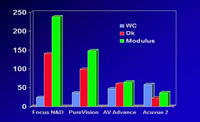 |
| Figure 2 - click to enlarge |
pyrrolidone (PVP).(9) Figure 2 graphically indicates
the inverse relationship between water content of hydrogel materials
and oxygen permeability and material stiffness, with the materials
having the highest ratio of silicone to water being the stiffest.
Increased rigidity or stiffness has some advantages, in that
the lenses handle very well. Increased rigidity might also suggest
more corneal astigmatism is masked compared to flexible hydrogels,
but that has not been our experience clinically or that of others.(10)
The mechanical properties of these lenses do pose some problems,
in that they are less able to conform easily to the shape of the
eye and fitting is critical, with loose lenses exhibiting poor
comfort.(11) Additionally, the rigidity of these materials may
be implicated in a variety of mechanical complications seen with
silicone-hydrogel lenses, including papillary conjunctivitis and
superior epithelial splits.(12-17)
Dehydration
Dry eye symptoms are reported by 20-50% of soft lens wearers,(18-19)
with 35% of patients permanently ceasing lens wear due to complications
associated with discomfort and dryness.(20)
The sensation of “dryness” is a complex subject and
is without question related to a variety of factors. One factor
to consider is that of lens dehydration, as the subjective symptom
of dryness appears to occur more frequently in soft lens wearers
whose lenses undergo greater dehydration during open-eye wear.(21)
Material composition influences dehydration rate and degree.(22)
In a clinical environment it has been noted that the majority
of wearers of silicone hydrogel lenses report that their lenses
feel less “dry” than their previous conventional lenses,
despite considerably longer wearing times.(23) These novel materials,
which have lower water contents than currently available materials,
may produce less subjective dryness symptoms through reduced in-eye
dehydration, enhanced wettability, reduced hydrophobic interactions
with the eye-lid, reduced deposition and/or increased oxygen performance.
Published work to-date shows that silicone-hydrogel lens materials
dehydrate at a slower rate and to a lesser extent than conventional
hydrogel materials(24,25) and may partially help to explain this
reduction in the sensation of dryness.
Surface Properties
Surface Wettability
Historically, a huge impediment to the development of silicone
hydrogel lenses has related to the decreased wettability, increased
lipid interaction and accentuated lens binding inherent in silicon-based
materials, as previously described. In order to render the surfaces
hydrophilic, techniques incorporating plasma into the surface
processing of the lens have been developed.(26-29) The purpose
of this surface treatment is to mask the hydrophobic silicone
from the tear film, increasing the surface wettability of the
materials and reducing lipid deposition.
The surfaces of Focus Night & Day lenses are permanently
modified in a gas plasma reactive chamber to create a permanent,
ultrathin (25 nm), high refractive index, continuous hydrophilic
surface.27,30,31 PureVision lenses are surface treated
in a gas plasma reactive chamber which
 |
| Figure 3 - click to enlarge |
transforms the silicone components on the surface of the lenses
into hydrophilic silicate compounds.(26,29,32-34) Glassy, island-like,
discontinuous silicate “islands” result,(33) and the
hydrophilicity of these areas "bridges" over the underlying
hydrophobic balafilcon A material. The subtle differences in the
surfaces of these novel materials can be clearly appreciated using
very high magnification imaging techniques such as atomic force
microscopy (AFM) (Figure 3).
Both surface treatments become an integral part of the lens and
are not surface coatings that can be easily “stripped”
away from the base material during daily handling and cleaning.
The Acuvue Advance material is the first non surface-treated
silicone hydrogel to become a commercial reality. Acuvue Advance
uses an internal wetting agent (Hydraclear™) based upon
PVP, which is designed to provide a hydrophilic layer at the surface
of the material that “shields” the silicone at the
material interface, thereby reducing the degree of hydrophobicity
typically seen at the surface of siloxane-hydrogels.(35-38) Details
on the Advance material are scanty thus far, but some information
can be gleaned from patents issued or pending. The silicone content
is derived from a tailor-made silicone macromer, used in conjunction
with TRIS monomer, copolymerised with hydrogel-forming monomers
such as N,N-dimethyl acrylamide and HEMA, in the presence of around
5% of PVP and an organic diluent together with a small amount
of cross-linking agent. Extraction of the diluent and hydration
leads to a silicone hydrogel which is sufficiently wettable to
avoid the need for subsequent surface treatment.
Analysis of the surfaces of both PureVision and Focus Night
& Day has shown that these surface treatments have only been
partially effective at masking the silicone, with the lenses having
significantly more silicon exposed at the surface than conventional
lenses(39) and a more hydrophobic surface.(40,41) No details yet
exist on the amount of silicon exposed on the Advance surface.
Lipid and Protein Deposition
It is important that silicone hydrogel materials do not deposit
to the degree that silicone elastomer lenses did as these lenses
are primarily intended for overnight use for up to 30 days and
such deposition would require frequent lens removal for cleaning.
To date, the degree of in-eye biocompatibility achieved with silicone-hydrogel
materials has received relatively minimal attention, with the
published results indicating that the deposition of protein on
these materials is less than that seen with conventional materials,(42-47)
but that lipid deposition can be a problem for certain patients,(44)
particularly if they are refitted from an ionic material such
as etafilcon that deposits very little lipid. If subjects are
seen to be depositing their lenses with lipid then moving to non-NVP-containing
materials (such as Proclear or Acuvue) will reduce lipid deposition.
Further options include adding surfactant cleaners containing
alcohol (such as Miraflow) or moving to more frequent periods
of replacement.(48)
What of the future? Increasingly,
contact lens companies are looking at developing novel silicone-based
hydrogels and the foreseeable future for this group of lens materials
looks promising, with several other new hydrogel materials already
registered with the USAN Council. Whilst the exact details of
such materials (eg acquafilcon, lenefilcon and senofilcon) are
unknown, it is likely that the next 10 years will be dominated
by the release of silicone-based hydrogels from all manufacturers.
These materials will likely have stiffness levels closer to conventional
hydrogels and have better surface treatments that truly make the
surfaces hydrophilic. Ideally, such materials would support a
tear film for longer than the typical 7-8 seconds seen with currently
available materials and consist of polymers that would resist
contamination with pathogenic organisms.(49) Such materials would
result in increased comfort and reduced inflammatory complications
compared with currently available materials and would have a significant
impact on growing the contact lens market.
References
- Papas E, Vajdic C, et al.: High oxygen-transmissibility
soft contact lenses do not induce limbal hyperaemia.
Curr Eye Res 1997; 16;9: 942-948.
- Dumbleton KA, Chalmers RL, et al.: Changes in myopic
refractive error with nine months' extended wear of hydrogel
lenses with high and low oxygen permeability. Optom
Vis Sci 1999; 76;12: 845-849.
- Dumbleton KA, Chalmers RL, et al.: Vascular response
to extended wear of hydrogel lenses with high and low oxygen
permeability. Optom Vis Sci 2001; 78;3: 147-151.
- Dumbleton K: Adverse events with silicone hydrogel continuous
wear. Contact Lens & Ant Eye 2002; 25 137 - 146.
- du Toit R, Simpson TL, et al.: Recovery from hyperemia
after overnight wear of low and high transmissibility hydrogel
lenses. Curr Eye Res 2001; 22;1: 68-73.
- Keay L, Sweeney DF, et al.: Microcyst response to
high Dk/t silicone hydrogel contact lenses. Optom Vis
Sci 2000; 77;11: 582-585.
- Fonn D, du Toit R, et al.: Sympathetic swelling
response of the control eye to soft lenses in the other eye.
Invest Ophthalmol Vis Sci 1999; 40;13: 3116-3121.
- Covey M, Sweeney DF, et al.: Hypoxic effects on
the anterior eye of high-Dk soft contact lens wearers are negligible.
Optom Vis Sci 2001; 78;2: 95-99.
- Steffen R, Schnider C: A next generation silicone hydrogel
lens for daily wear. Part 1 - Material properties.
Optician 2004; 227;5954: 23 - 25.
- Edmondson L, Edmondson W, et al.: Masking astigmatism:
Ciba Focus Night and Day vs Focus monthly. Optom Vis
Sci 2003; 80;12s: 184.
- Dumbleton KA, Chalmers RL, et al.: Effect of lens
base curve on subjective comfort and assessment of fit with
silicone hydrogel continuous wear contact lenses. Optom
Vis Sci 2002; 79;10: 633-637.
- Holden BA, Stephenson A, et al.: Superior epithelial
arcuate lesions with soft contact lens wear. Optom
Vis Sci 2001; 78;1: 9-12.
- Jalbert I, Sweeney DF, et al.: Epithelial split
associated with wear of a silicone hydrogel contact lens.
CLAO J 2001; 27;4: 231-233.
- O'Hare N, Naduvilath T, et al.: A clinical comparison
of limbal and paralimbal superior epithelial arcuate lesions
(SEALs) in high Dk EW. Invest Ophthalmol Vis Sci 2001;
42;4: s595.
- Skotnitsky C, Sankaridurg PR, et al.: General and
local contact lens induced papillary conjunctivitis (CLPC).
Clin Exp Optom 2002; 85;3: 193-197.
- Jones L, Dumbleton K: Silicone hydrogel lenses: Fitting procedures
and in-practice protocols for continuous wear lenses.
Optician 2002; 223;5840: 37 - 45.
- Dumbleton KA, Jones L: Silicone hydrogel lenses: Follow-up
and management. Optician 2002; 223;5845: 34 - 43.
- Orsborn G, Zantos S: Practitioner survey: Management of dry
eye symptoms in soft lens wearers. Contact Lens Spectrum 1989;
4;9: 23-26.
- Doughty MJ, Fonn D, et al.: A patient questionnaire
approach to estimating the prevalence of dry eye symptoms in
patients presenting to optometric practices across Canada.
Optom Vis Sci 1997; 74;8: 624-631.
- Weed K, Fonn D, et al.: Discontinuation of contact
lens wear. Optom Vis Sci 1993; 70;12s: 140.
- Efron N, Young G: Dehydration of hydrogel contact lenses
in vitro and in vivo. Ophthal Physiol Opt
1988; 8;3: 253 - 256.
- Efron N, Morgan P: Hydrogel contact lens dehydration and
oxygen transmissibility. CLAO J 1999; 25;3: 148 - 151.
- Fonn D, Pritchard N, et al.: Factors affecting
the success of silicone hydrogels. in Silicone Hydrogels:
The Rebirth of Continuous Wear Contact Lenses, D. Sweeney,
Editor. Oxford, UK, Butterworth-Heinemann, 2000, pp 214 - 234.
- Jones L, May C, et al.: In vitro evaluation
of the dehydration characteristics of silicone-hydrogel and
conventional hydrogel contact lens materials. Contact
Lens & Ant Eye 2002; 25 147 - 156.
- Morgan PB, Efron N: In vivo dehydration of silicone hydrogel
contact lenses. Eye Contact Lens 2003; 29;3: 173-176.
- Grobe G, Kunzler J, et al.: Silicone hydrogels for
contact lens applications. Polymeric Materials Science
and Engineering 1999; 80 108 - 109.
- 27. Nicolson PC, Vogt J: Soft contact lens polymers: an evolution.
Biomaterials 2001; 22;24: 3273-3283.
- Nicolson PC: Continuous wear contact lens surface chemistry
and wearability. Eye Contact Lens 2003; 29;1 Suppl:
S30-32; discussion S57-39, S192-194.
- Tighe B: Silicone hydrogels: Structure, properties and
behaviour. in Silicone Hydrogels: Continuous Wear Contact
Lenses, D. Sweeney, Editor. Oxford, Butterworth-Heinemann,
2004, pp 1 - 27.
- Nicolson P, Baron R, et al.: Extended wear ophthalmic
lens. CIBA Vision; CSIRO, US Patent # 5,760,100. 1998.
- Weikart CM, Matsuzawa Y, et al.: Evaluation of plasma
polymer-coated contact lenses by electrochemical impedance spectroscopy.
J Biomed Mater Res 2001; 54;4: 597-607.
- Valint PL, Jr., Grobe GL, 3rd, et al.: Plasma surface
treatment of silicone hydrogel contact lenses. Bausch
& Lomb, US Patent # 6,193,369. 2001.
- Lopez-Alemany A, Compan V, et al.: Porous structure
of Purevision versus Focus Night & Day and conventional
hydrogel contact lenses. J Biomed Mater Res (Appl Biomat)
2002; 63 319 - 325.
- Tighe B: Soft lens materials. in Contact Lens
Practice, N. Efron, Editor. Oxford, Butterworth-Heinemann,
2002, pp 71 - 84.
- Vanderlaan D, Turner D, et al.: Soft contact lenses.
Johnson & Johnson, US Patent # 20020107324. 2002.
- Maiden A, Vanderlaan D, et al.: Hydrogel with internal
wetting agent. Johnson & Johnson Vision Care, US
Patent # 6,367,929. 2002.
- McCabe K, Molock F, et al.: Biomedical devices containing
internal wetting agents. Johnson & Johnson, US
Patent # 20030162862. 2003.
- McCabe K, Molock F, et al.: Biomedical devices containing
internal wetting agents. Johnson & Johnson, US
Patent # 20030125498. 2003.
- Karlgard C, Sarkar D, et al.: Drying methods for
XPS analysis of PureVision, Focus Night&Day and conventional
hydrogel contact lenses. Appl Surface Sci 2004; 230
106 - 114.
- Jones L, Long J, et al.: The impact of contact lens
care regimens on the in vitro wettability of conventional
and silicone-hydrogel contact lens materials. Invest
Ophthalmol Vis Sci 2002; ARVO abstract # 3097.
- Cheng L, Muller SJ, et al.: Wettability of silicone-hydrogel
contact lenses in the presence of tear-film components.
Curr Eye Res 2004; 28;2: 93-108.
- Senchyna M, Jones L, et al.: Optimization of methodologies
to characterize lysozyme deposition found on balafilcon and
etafilcon contact lens materials. Invest Ophthalmol
Vis Sci 2002; ARVO abstract # 3082.
- Senchyna M, Jones L, et al.: Quantitative and conformational
characterization of lysozyme deposited on balafilcon and etafilcon
contact lens materials. Curr Eye Res 2004; 28;1: 25-36.
- Jones L, Senchyna M, et al.: Lysozyme and lipid
deposition on silicone hydrogel contact lens materials.
Eye Contact Lens 2003; 29;1 Suppl: S75-S79.
- McNally J, McKenney CD: A clinical look at a silicone hydrogel
extended wear lens. Contact Lens Spectrum 2002; 17;1:
38 - 41.
- Bruinsma GM, van der Mei HC, et al.: Bacterial adhesion
to surface hydrophilic and hydrophobic contact lenses.
Biomaterials 2001; 22;24: 3217-3224.
- McKenney C, Becker N, et al.: Lens deposits with
a high Dk hydrophilic soft lens. Optom Vis Sci 1998;
75;12s: 276.
- Jones L, Mann A, et al.: An in vivo comparison
of the kinetics of protein and lipid deposition on group II
and group IV frequent-replacement contact lenses. Optom
Vis Sci 2000; 77;10: 503-510.
- Baveja JK, Willcox MD, et al.: Furanones as potential
anti-bacterial coatings on biomaterials. Biomaterials
2004; 25;20: 5003-5012.
Continued
< Part 1
|