A slitlamp examination of a patient complaining of sore, red eyes and variable vision uncovered multiple contact lens related conditions. Although overwear of contact lenses is a contributing factor in this case, it is not a diagnosis in itself.
A 35-year-old male presented on referral from a local optometrist. He reported sore, red eyes and variable vision for the previous two months and was wearing hydrogel contact lenses that had been prescribed in the United Kingdom about one year before. He was wearing the contact lenses all waking hours but denied sleeping in them.
The referring optometrist had advised the patient to discontinue contact lens wear but the patient continued wearing them part time and had noticed some improvement in his symptoms over the past week. The patient reported being in good health.
At presentation, unaided vision was R 6/19- L 6/24. The patient’s current spectacles were R -0.25/-2.75x85 L -0.75/-1.50x80 giving visual acuity of R 6/9.5 L 6/15. Pupils were equal and reactive with no afferent pupil defect noted. Ocular motility was full and visual fields were full to confrontation. Colour vision was intact in each eye to Ishihara plates. Subjective refraction was R -0.25/-3.00x95 (6/9.5) L -1.50/-4.00x85 (6/12-). Pinhole visual acuity was R 6/7.5- L 6/6-.
 |
 |
 |
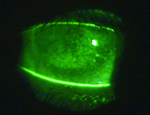
| Figure 1, click to enlarge |
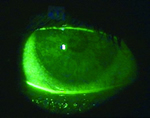
Figure 2, click to enlarge |
Figure 3, click to enlarge |
Slitlamp examination found severe superficial punctate keratitis left more so than right (Figures 1 and 2). The left cornea had a mid-peripheral infiltrate (Figure 3). Anterior chambers were quiet in each eye. There was extensive corneal limbal neovascularisation and bulbar conjunctival injection in both eyes (Figures 4 and 5). Inferior fornices and upper lid eversion found papillary conjunctivitis (Figures 6 and 7).
Although overwear of contact lenses is a contributing factor in this case, ’contact lens overwear‘ is not a diagnosis in itself. This patient had multiple conditions: contact lens induced papillary conjunctivitis, severe punctate keratitis (possibly infective) corneal infiltrate and corneal hypoxia.
Contact lens induced papillary conjunctivitis is thought to be an immunological response to denatured tear film protein that deposits onto the lens surface during wear. Infective punctate keratitis can be subdivided into two types: limbal and paracentral/central. It is important in the limbal subtype to carefully examine the corneal limbus for small infiltrates and this will aid the diagnosis. They often appear in between the injected limbal vessels.
The paracentral/central subtype (as in this patient) is potentially vision threatening if it progresses to a corneal ulcer. The distinction between a pure inflammatory reaction to surface bacteria and infective keratitis can be difficult to distinguish clinically. There is a high ocular surface bacterial load as indicated by the corneal infiltrate.
In a contact lens patient with a compromised cornea it was important to treat this condition appropriately with a fluoroquinolone to prevent possible progression to a corneal ulcer. This is not to treat the infiltrate itself, but to remove the stimulus (high bacterial load and/or infected epithelium) causing the infiltrate.
Once the ocular surface bacterial load has been decreased, topical anti-inflammatory treatment will be needed to suppress the corneal and conjunctival immunological response. Contaminated contact lenses, solutions and cases are often the source of the bacterial infection and the patient should be advised to discard these.
The patient was prescribed gtt. ofloxacin 0.3% (Ocuflox) q6d, told to discontinue all contact lens wear and scheduled for review in five days due to it being the day before the practice closed for Christmas. He was given an emergency mobile telephone number to contact if symptoms increased.
At review the patient reported good compliance with the prescribed treatment. He reported his eyes felt much better. Visual acuity with his glasses was R 6/7.5+ L 6/7.5-. Slitlamp examination found improvement in the superficial punctate keratitis and conjunctival injection (Figures 8 and 9). The papillary conjunctivitis and corneal infiltrate appeared unchanged.
The patient was told to alter the dosage of gtt. ofloxacin 0.3% (Ocuflox) to qid and prescribed gtt. fluorometholone acetate (Flarex) qid. Fluorometholone acetate is indicated to treat the corneal infiltrate and contact lens papillary conjunctivitis. Ofloxacin is continued to give antibiotic cover while the patient is on topical immunosuppressant treatment. Review was scheduled for six days.
At the next review the patient reported his eyes were feeling much better and his vision was less variable. Visual acuity with his spectacles was R 6/7.5+ L 6/4.8-. Slitlamp examination found only trace superficial punctate keratitis in the right eye and more significant but improving superficial punctate keratitis in the left eye (Figures 10 and 11). The limbal injection had resolved (Figure 12) and the papillary conjunctivitis was improving (Figure 13 and 14). Intraocular pressures were R 14 L 14.
The patient was told to continue gtt. ofloxacin 0.3% (Ocuflox) qid and gtt. fluorometholone acetate (Flarex) qid and prescribed unit dose gtt. ketotifen (Zaditen) bid. The ketotifen is added here to address the more chronic nature of contact lens induced papillary conjunctivitis as the fluorometholone acetate was to be discontinued shortly. Due to the multiple topical medications prescribed, the non-preserved unit form of ketotifen was preferred to reduce corneal toxicity. Review was scheduled for seven days.
The next review was now 18 days after the first presentation. The patient had finished the gtt. ofloxacin 0.3% (Ocuflox) two days earlier. He reported his eyes felt normal. Visual acuity with spectacles was R 6/6- L 6/6-. Intraocular pressures were R 14 L 14. Dilated fundus examination was unremarkable.
Slitlamp examination found no corneal fluorescein staining, a small mid-peripheral cornea scar at the site of the original infiltrate, ghosted limbal neovascularisation vessels and only minor papillary conjunctivitis (Figures 15-17). A fast taper of the gtt. fluorometholone acetate (Flarex) was started and gtt. ketotifen (Zaditen) bid was continued. Review was scheduled for seven days.
At the next review the patient reported his eyes felt good. Visual acuity with spectacles was R 6/7.5 L 6/6. Slitlamp examination found no corneal fluorescein staining, a small almost faded mid-peripheral cornea scar at the site of the original infiltrate, ghosted limbal neovascularisation vessels and only minor papillary conjunctivitis. Corneal topography demonstrated marked changes from the initial presentation back to baseline (Figures 18 and 19). The patient was advised to continue gtt. ketotifen (Zaditen) bid for three months and not to wear contact lenses for three months.
The patient was referred back to the original optometrist for ongoing care with the recommendation of changing to silicone hydrogel contact lenses to address corneal hypoxia and a review of care and maintenance procedures if contact lens wear was recommenced.
The author thanks Dr Erwin Groeneveld for his advice in preparation of this article.
The Silicone Hydrogels Editorial Board also thank the Optometry Pharma Journal for allowing us to reproduce this article.
|