Introduction: silicone hydrogels - the rebirth of silicon as an ophthalmic material
The last decade has been very significant for the development of contact lens materials. The most important feature of this period has been the commercial appearance of the so-called silicone hydrogels, which differ from conventional hydrogels in their enhanced oxygen permeabilities. This, in turn, is a direct consequence of the inclusion of a significant proportion of siloxy groups (which.contain the element silicon linked directly to both oxygen and carbon atoms). In this way silicon has re-emerged as a vitally important element in ophthalmic biomaterials. The first material to be used for such applications was, of course, glass, which is predominantly silicon dioxide, but this was progressively displaced by optically clear synthetic carbon-based polymers, notably polymethyl methacrylate (PMMA). The fact that the element silicon is once more growing in importance in ophthalmic biomaterials is substantially unrelated to the factors that underpin its important place in materials history: its ability to form clear silicate glasses and its natural abundance. Nor is its use inspired by the way in which it performs in the human body – where it is largely absent. It is instead dependent upon the uniquely high oxygen permeability of long molecular chains (polymers) based on the siloxy group described above, which in its most commonly encountered form is known as silicone rubber. Because oxygen is so vital to the human body and because the oxygen permeability of silicone rubber and related compounds is much higher than that of any natural material, they have tremendous potential in the medical device field. There have been many attempts over the last forty years to harness the softness and oxygen permeability of silicone rubber in the contact lens field, but it was not until the commercial appearance of silicone hydrogel lenses, in 1998, that any significant success was achieved.
The changes that the introduction of silicone hydrogels has brought about in the contact lens field have surprised even their most enthusiastic advocates. In the first place the silicone hydrogel market has shown tremendous year on year growth. Because of their enhanced oxygen permeability they were developed primarily with extended wear in mind, but they have proved attractive to practitioners for both overnight and daily wear modalities. Coupled with this growth and dual usage has been the progressive development of silicone hydrogel technology. The dual modality has provided a platform for improvements in all three critical contact lens material properties – permeability behaviour, surface properties and mechanical properties (e.g. modulus) – rather than oxygen permeability alone. At the time of writing there are six commercial materials and more known to be in the pipeline. Between them these cover water contents from 24% to 48%, oxygen permeabilities (Dk) from 60 Barrers to 140 Barrers, initial moduli (stiffness) from 0.4 to 1.4 MPa and surface treatments ranging from plasma oxidation and coating, through entrapped polyvinyl pyrrolidone (PVP) to none at all. Table 1 summarises the relevant information.
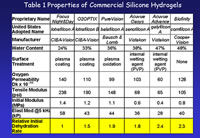 |
Table 1 |
click to enlarge |
The purpose of this editorial is to outline the development of silicone hydrogels, and to highlight for practitioners the factors that underpin the progressive changes that are taking place. Because the chemical structures and patent information are readily available in other reviews, and because most practitioners have been regularly bombarded with such information, a substantially descriptive approach will be adopted here.
The Routes to Success: Tris and Silicone Macromers
The fact, referred to above, that silicone rubber has such high oxygen permeability is the single feature that has driven the development of commercial silicone hydrogels – as it did the earlier development of RGPs. Despite attempts to harness the outstanding oxygen permeability and resilience of silicone rubber, it was not until the development of the silicon-containing “Tris” monomer, and its use to convert PMMA into gas permeable rigid lenses, that silicon made any real commercial impact in ophthalmic polymers. Once technically and economically viable solutions to the problem of incorporating essentially the same Tris monomer into hydrogels had been found, and the market was judged to be ready to embrace the concept of extended wear, silicon began to play a new and important role in the field of ophthalmic biomaterials. The fact that twenty years elapsed between the publication of first patent (Tanaka) that contained an indication of how the structure of Tris monomer could be modified to make it compatible with hydrogels and the widespread launch of silicone-hydrogel contact lenses illustrates the point that the problems are more complex than might have been initially assumed. This conclusion is further supported by the fact that additional approaches to silicone hydrogel formation have been regularly described in the patent literature both before and after the launch of the first commercial products. It would be useful to describe briefly the features that distinguish the products that have progressively emerged in the silicone hydrogel field and ask why there has been a progressive evolution of properties and whether such evolution can continue.
So what was the answer to the problem of combining the elements of silicone rubber, or the siloxy monomer commonly referred to as TRIS – both of which are hydrophobic - with hydrophilic monomers such HEMA or NVP to form silicone hydrogels. The same fundamental difficulty exists as in trying to combine oil and water to form an optically clear product. Phase separation occurs and the optical clarity of the product is impaired. Phase separation is only a visual problem if the size of the discrete phases is of the order of the wavelength of light or greater. Some degree of segregation probably occurs in all silicone hydrogels and has been used as a positive benefit in products such as Ciba’s Focus Night&Day.
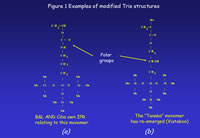
Two broad groups of solutions to the incompatibility problems have been exemplified in patents, since the late 1970s. The approach that was first used involves the insertion of polar groups into the stem of the Tris molecule to aid its miscibility with hydrophilic components. The second approach is the development of macromer technology. Macromers are large monomers formed by pre-assembly of structural units that are designed to bestow particular properties on the final polymer. The macromers used to form silicone hydrogels contain structural units taken from the silicone rubber backbone, often interspersed with polyethylene glycol (PEG) sequences to provide the hydrophilicity. Examples of modified Tris molecules are shown in Figures 1a and 1b; an example of a siloxy macromer is shown in Figure 2.
 |
 |
Figure 1a, 1b |
Figure 2 |
click to enlarge
|
These approaches (which are not mutually exclusive) both reflect what has been the major thrust of most silicone hydrogel patents, the development of compositions based on silicone-containing monomers that are sufficiently compatible with a range of hydrophilic monomers to enable hydrogels with high loadings of silicone to be achieved. The advantage of incorporation of siloxy groups is illustrated schematically in Figure 3.
 |
Figure 3 |
click to enlarge |
As we make use of this diagram in interpreting the Dk of the range of commercial products it is important to two factors relating to achievable values of Dk. Firstly, that the Dk of water itself is only around 100 Barrers, and secondly, that the oxygen permeability of a polymer entirely composed of Tris (Dk around 175 Barrers) is only about one third of that achievable with silicone rubber. The consequence is that higher oxygen permeabilities are achievable with silicone hydrogels prepared from macromers (which are based on silicone rubber segments) than those based on modified Tris structures. An examination of the products shown in Table 1 will illustrate this and point the way to likely future commercial developments.
PureVision and Night&Day: The First Commercial Materials
The first two products to be launched commercially were Focus Night&Day and PureVision.which were launched within months of each other. Examination of the patents that led up to and underpin these products reveal that Bausch & Lomb have concentrated on the “modified Tris” approach outlined above, and developed a range of novel monomers, typified by the monomer that forms the basis of PureVision (Figure 1a). The CibaVision approach, on the other hand, has been more focused on macromers containing silicone rubber sequences (e.g. Figure 2) and polymerisation methodologies to combine these with unmodified Tris.
As Table 1 shows, Focus Night&Day (lotrafilcon A) is the stiffest and most oxygen permeable (having a DK of 140 Barrers) of the commercial materials. This is a consequence of its relatively low water content (24%). In order to maintain adequate on-eye movement Ciba harnessed the inherent tendency of Tris and hydrophilic components to phase separately in an intriguing way that produced co-continuous phases that enable both high oxygen permeability and high water and sodium ion permeability to be achieved. In their patent describing this effect they define minimum levels of ion or water permeation that are required to enable the lens to move adequately on the eye. The data contained in the Ciba patent predict that once the water content reaches the mid-thirties, the inherent aqueous and ionic permeability of the gel will be adequate to maintain on-eye lens mobility.
Pure Vision (balafilcon A) is based on a substantially homogeneous copolymer of a vinyl carbamate derivative of TRIS (Figure 1a) giving a water content of 35% and a DK of 110 Barrers. It is said to have a water transport slightly (10%) in excess of that of polyHEMA (Salamone, Grobe, Seelye and Künzler, 1999). This would put it above the critical minimum value of ionic and hydraulic permeability for lens movement on eye as defined in the Ciba patent. It may be that there is some degree of phase separation in the material to account for the fact that the water transport value is enhanced relative to its actual water content. In any event the chosen water content of 35% gives optimal oxygen permeability consistent with adequate water permeability to maintain lens movement on eye.
One final feature that must be highlighted is the fact that the first two commercial silicone hydrogels, Pure Vision and Focus Night & Day, are modified by a surface treatment process carried out after the lens has been fabricated. Both are treated using gas plasma techniques but whereas Bausch & Lomb have opted for plasma oxidation, Ciba have chosen to apply a plasma coating. In the former case (Pure vision) oxidation of TRIS produces hydrophilic glassy silicate islands on the surface, whereas the surface of Night & Day is coated with a 25 nm thick, dense, high refractive index coating.
Phase Two: Higher Water Contents and Internal Wetting
Much of the commercial realisation of this silicone hydrogel technology and especially the timing of product launches has been governed by patent ownership and litigation. Although Tanaka’s monomer (Figure 1b) was the first monomer of this type to be exemplified in the patent literature it was not converted into a commercial silicone hydrogel in the lifetime of the patent. It formed the basis of not the first, but the third commercial silicone hydrogel lens. Tanaka’s initial intent seems to have been to use it in rigid gas permeable materials, primarily in an attempt to improve surface properties, although there is no evidence that it was successful in that role. Its use in silicone hydrogels did not come until the original 1979 patent had expired twenty-five years later. Workers at Vistakon developed a much-improved synthesis for the Tanaka monomer and were thus able to use it as a key component of Acuvue Advance (galyfilcon-A) which was launched in 2004 upon the expiry of the original patent, followed by a higher Dk material (Acuvue Oasys, senofilcon-A). Both materials utilize the Tanaka monomer in conjunction with a siloxy macromer and hydrophilic monomers such as HEMA and N, N- dimethyl acrylamide (DMA). Acuvue Oasys has a water content of 38% and a Dk of 103Barrers. whilst Acuvue Advance has the highest water content of the group (47%) and the lowest Dk (60 Barrers) and, as a consequence is only approved for daily wear.
The appearance of Advance and Oasys was significant in relation to both surface and mechanical properties. They were the first commercial materials that did not employ a surface treatment as a separate step after fabrication of the lens. This has the advantage of avoiding possible patent infringement and reducing manufacturing costs. Both Acuvue Advance and Acuvue Oasys use an internal wetting agent (Hydraclear™), which is high molecular weight polyvinyl pyrrolidone (PVP). This is incorporated into the lens material at the lens fabrication stage and is designed to provide a hydrophilic layer at the surface of the material that “shields” the silicone at the material interface. The technique appears to be successful and to work well in terms of wettability, lubricity and clinical acceptability.
The second significant feature of Advance and Oasys was their mechanical properties. The first two commercial silicone hydrogel lenses were much “stiffer” than their conventional hydrogel counterparts. This is apparent from inspection of Table 1. The stiffness of the materials is here compared in three different ways. The tensile modulus is measured by pulling or extending a strip of the lens material until it breaks and a measure of the stiffness as it is being stretched. The initial modulus is similar, but is measured only during the first few percent of the deformation process – more akin to the deformation during handling. The elastic modulus is measured as the lens is being rapidly subjected to a slight deformation and allowed to recover before being deformed again – as it would be during the blink. This allows the tendency of the material to either recover or to flow to be measured. The force required to deform the material combined with the ability of the material to recover elastically is converted to the elastic modulus. From the figures quoted it can be seen that the relative stiffness of a polymer depends upon how the material is deformed, reflecting the different ways in which the material is deformed during use.
Whichever method is used, the stiffness of the first two silicone hydrogel materials, Focus Night&Day and PureVision, is found to be several times greater than low rigidity conventional materials such as etafilcon A. The silicone hydrogel material with the highest water content and lowest Dk, Acuvue Advance, has a modulus that is much closer to conventional materials, being only around 1.5 times more rigid than etafilcon A. The major initial impact made by the two Vistakon products related to the fact that their stiffness was so much less than that of Night&Day and PureVision. It seems very likely that the introduction of O2OPTIX (now AIROPTIX) which is essentially a lower modulus, higher water content version of Night&Day was a response to that impact. These comparisons illustrate how important water content is in determining properties such as stiffness and oxygen permeability for materials of similar structure. This point is readily illustrated by reference to Table 1 in which materials have been arranged in order of decreasing water content. We can extend this analysis to the points that have already been made about the intrinsic difference in Dk between silicone rubber and polyTris and the effect that this has on the balance between water content and Dk.
Phase Three: Breaking the Dk Paradigm
Inspection of Figure 3 shows five crosses, corresponding to the materials described so far. As already explained the silicone hydrogel points (black dots) relate to silicone hydrogels based on Tris structures. PolyTris has a Dk of around 175 Barrers and this corresponds to zero water content and, of course, zero hydrophilic monomer content. Of the materials described only PureVision is based solely on a modified Tris structure as the source of Silicon. This material gives the lowest Dk for its water content and thus lies almost on the “theoretical” line. The other four materials are based on mixtures of silicone rubber-based macromers and Tris or modified Tris structures. The detailed structures and ratios are different for each material, but there is a remarkably good water content / Dk inter-relationship. The logical question is this. If we abandon Tris-based structures and make silicon hydrogels based solely on macromers as the source of silicon, can we achieve higher Dkvalues at a given water content?
We need to remember that macromers vary considerably in structure, but the point has been demonstrated for us by the appearance of the comfilcon material, which is solely macromer-based with no Tris derivatives. This is the final entry in the Table and is represented in Figure 3 by a circle. It is clear that the Dk value lies well above the notional line that passes through the crosses and thus has a superior Dk/water content relationship to all the materials that contain Tris structures.
The comfilcon (Biofinity) material is interesting for two reasons. The first is the fact that the oxygen permeability is, on the basis of existing materials, unexpectedly high for its water content. The second is the absence of either surface treatment or an internal wetting agent.– as inspection of Table 1 shows.
The technology underpinning the comfilcon material originates in a Japanese patent filed in December 2000 by the Asahikasei Aime Co., Ltd. In 2001 Ocular Sciences and the Asahikasei Aime Company entered into a joint Research and Development Relationship. Under the terms of the agreement, Ocular Sciences was to apply its cast moulding manufacturing process to the newly developed silicon-based material created by Asahikasei Aime to produce a “state of the art, high performance contact lens”. Following the coming together of Ocular Sciences and Coopervision a further patent was filed naming a Coopervision co-inventor.
There are interesting disclosures in the patent, which give a clue as to the reasons for the departure of comfilcon from the previous “mould” in which silicone hydrogels had been developed. The first is the fact that the conventional “Tris” monomer and its derivatives are not used. Instead the patent claims that two siloxy macromers of different sizes, one of which is only monofunctionalised (contains only one polymerisable double bond) when used together produce advantageously high oxygen permeabilities. The patent contains other subtleties, some relating to particular hydrophilic monomers, which, taken together, appear to have enhanced the compatibility of the silicone moieties with the hydrophilic domains. This explains the absence of need for internal wetting agents or surface treatment and produces a very useful addition to the silicone hydrogel product portfolio.
Although higher water contents bring advantage in terms of mechanical properties there is an area where they bring disadvantage.. A major problem with contact lenses continues to be their reduction in perceived comfort over the wearing period, particularly as the lens surface dehydrates. The sensation of “dryness” is a complex subject and is without question related to a variety of factors of which lens dehydration is one of the most important. The final highlighted row of Table 1 shows that dehydration rates, as measured by dynamic vapour sorption, rise with increasing water content. Although the majority of wearers of silicone- hydrogel lenses report that these lenses feel less “dry” than conventional lenses it may simply reflect the fact that the water contents of silicone hydrogels are generally lower than conventional hydrogels. There is no comparative evidence involving conventional and silicone hydrogels at matched water contents.
Future Opportunities
Although there has been considerable progress in novel silicone-based hydrogels over the last few years there is still much to be achieved. There is scope for considerable gain in Dk at given water content, using the principles outlined here. Surface properties are an obvious area for development, perhaps using the techniques of macromolecular entrapment and release currently being exploited in daily disposable lenses, and indeed, to a degree in Acuvue Advance and Oasys. The aim of such developments is logically enhanced comfort and tear film stability together with reduced lipid deposition and enhanced in-eye biocompatibility. There is considerable scope for parallel developments in care solutions, specifically for silicone hydrogels used in daily wear modality.
|