Introduction
The corneal epithelium is only 50 microns thick, about 1/10 the
thickness of a credit card, yet it plays a pivotal role in
protecting the eye against mechanical damage and the penetration
of infectious organisms into the cornea. Furthermore, the corneal
epithelium provides a stable underground for the anchoring
of the tear film, which contains additional defensive agents
against infection. Every day, cells on the surface of the epithelium
exfoliate into the tear film and are replaced by younger underlying
cells. To maintain this continuous process of renewal, a nonstop
need for new cells (proliferation) is required. Proliferation
exclusively occurs in the basal cell layer. In addition, there
is slow movement of cells towards the center (centripetal)
and/or the tear film; in this regard, the corneal epithelium
can be seen as a slow moving river of epithelial cells. The
continuous production and flow of epithelial cells is of vital
importance for the maintenance of the corneal epithelium and
its protective functions. It is not surprising therefore that
a partial breakdown of the corneal epithelium or a serious
alteration of its normal physiology may lead to a decrease
in effectiveness against infection.
Over the past three decades, we have learned a great deal about
the effects of daily (DW) and extended contact lens wear (EW)
on the corneal epithelium, as both wearing modalities are capable
of inducing various epithelial changes; some are minor, others
seem more serious. How does the new generation of soft lenses,
silicone-hydrogel lenses, fit into this picture? Do silicone-hydrogel
lenses affect the corneal epithelium? If yes, how do they compare
to conventional soft lenses? Does it make a difference whether
patients sleep 6 nights or 30 nights in silicone hydrogel lenses?
The aim of this editorial is to review our current understanding
of the effects of silicone hydrogel contact lens wear on the
corneal epithelium.
Changes of the corneal epithelium during EW
Silicone-hydrogel lenses
Central thickness
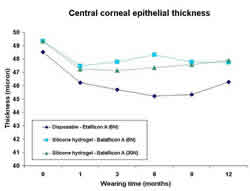
In a prospective, randomized, double masked study it was shown
that DW with silicone hydrogel test lenses (Balafilcon A) and
control lenses (Etafilcon A) do not significantly thin the central
corneal epithelium. 1 A similar study on daily wear with the
Lotrafilcon A silicone hydrogel lens and the Etafilcon A lens
revealed the same result.2 EW however, is capable of causing
significant thinning of the central corneal epithelium (figure
1). 2, 3 The Etalfilcon A lens, worn on a weekly disposable
basis, thinned the corneal epithelium -6.8% following the first
six months of EW. Thereafter, the corneal epithelium had partially
recovered as it was -4.6% thinner than baseline at conclusion
of the study. The Balafilcon A lens produced less thinning than
the control lens with overall thinning after 1 year of -2.9%
and -3.2% in the monthly and weekly replacement of Balafilcon
A lenses respectively. Importantly, there was no statistically
significant difference between the 6N and 30N groups. Comparable
conclusions were reported with the Lotrafilcon A silicone hydrogel
lens.2 In summary, lens oxygen transmissibility and not wearing
schedule seems to control the thinning response during hydrogel
lens wear.
 |
| Figure
1 - click to enlarge |
Surface cell size
Unlike DW with silicone hydrogel lenses1, EW causes a significant
increase in cell size of the superficial epithelial cells in
the central cornea. This cell enlargement over time appears to
be equal in patients wearing silicone and conventional hydrogel
lenses.2, 3 Analogous to the corneal epithelial thickness measurements,
there did not seem to be any difference in cell size changes
between 6N and 30N silicone hydrogel lens wear.
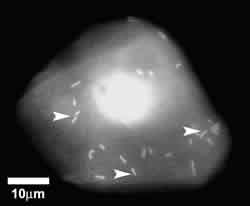
Pseudomonas aeruginosa (PA) - binding
Using an eye irrigation chamber, it is possible to collect surface
epithelial cells from human corneas. In a laboratory, these cells
can then be mixed with Pseudomonas aeruginosa bacteria
to assess bacterial adherence to individual cells (figure 2).
As it turns
out, corneal epithelial cells collected from contact lens patients
bind significantly more PA bacteria than non-lens wearing controls.
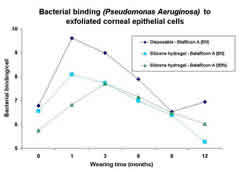
Silicone hydrogel lenses (DW and EW) have also shown increases
in PA-binding although not as great as current disposable hydrogel
lenses.2, 3 Interestingly, the highest PA-binding appeared to
be during the first 1-3 months of EW, thereafter, an adaptation
of the epithelium with less PA-binding in all test lens groups
occurred. Again, no significant difference between 6N and 30N
EW of silicone hydrogels was noted. (figure 3)
 |
 |
| Figure
2 - click to enlarge |
Figure 3 - click to enlarge |
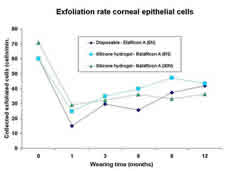
Exfoliation
The normal exfoliation rate of superficial corneal epithelial
cells in Etalfilcon A, Balafilcon A and Lotrafilcon A contact
lens wearing patients is known to decrease during both DW and
EW with no perceptible differences between silicone hydrogel
(6N and 30N) and control lenses (figure 4).1-3 These findings
correlate well with the results of several animal studies in
which similar decreases in surface cell death were observed with
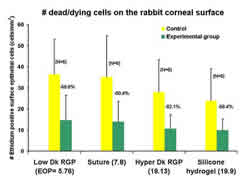
silicone hydrogel and other test lenses.4-6 Figure 5 shows the
distribution of dead/dying epithelial cells on the rabbit corneal
surface following several modes of contact lens wear. Overall,
it suggests that the physical presence of the contact lens rather
than the oxygen transmissibility of the lens material causes
the down-regulation of surface cell exfoliation. However, it
should be noted that collected cells from silicone hydrogel lens
wearers appear to be indistinguishable in size and viability
from non-lens wearers while cells collected from disposable low
Dk control lens wearers demonstrate a statistically significant
increase in diameter.7
 |
 |
| Figure
4 - click to enlarge |
Figure
5 - click to enlarge |
Proliferation
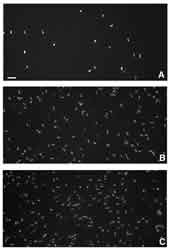
Cell Division in the corneal epithelium is greatly inhibited
during short-term low Dk EW. A contact lens with a Dk=15 for
example causes an average suppression of about -80% (figure 6).8
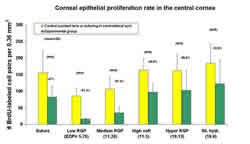
Figure 7 shows that of all test lenses worn continuously for
48-hours, silicone hydrogel lens wear (Balafilcon A) reduced
normal corneal epithelial cell division the least.9 The difference
in proliferation rates between low and high oxygen transmissible
hydrogel lenses may explain why central epithelial thinning is
more pronounced in the lower oxygen transmissible soft lenses
seen in clinical studies.2, 3 Preliminary, long term data however,
shows an increase in corneal epithelial proliferation during
continuous silicone hydrogel lens wear, suggesting a possible
adaptation of the corneal epithelium to the new conditions.9
It is not known yet if this also takes place with lower oxygen
transmissible lenses.
 |
 |
| Figure
6 - click to enlarge |
Figure
7 - click to enlarge |
Clinical, non-inflammatory observations:
mucin balls and microcysts
Epithelial microcysts and mucin balls have been described extensively
on this website. Briefly, the presence of epithelial microcysts
in the corneal epithelium is a good clinical indicator of chronic
hypoxia. Unlike disposable contact lenses, silicone hydrogel
lenses do not induce an increase in epithelial microcysts when
worn on EW basis.10-12 This is another piece of strong evidence
in favor of silicone hydrogel lenses suggesting that the problem
of hypoxia in the clinical practice can be referred to our contact
lens history books.
One clinical observation that appears to be
on the rise with silicone hydrogel lens wear is/are localized
depression(s) in
the corneal epithelium, associated with mucin balls.11, 13-17
It is believed that from time to time mucin balls can form and
become trapped between the corneal epithelium and the contact
lens (in the post-lens tear film). These ball-like structures
may then press themselves partially into the easily moldable
epithelial layer, causing spherical indentations as seen with
biomicroscopy 13, 14 and in vivo confocal microcopy (figure 8).16,
17 In an animal model it was shown that these spherical indentations
are distinct holes in the corneal epithelium lacking epithelial
cell nuclei.16 Care should be taken not to confuse these indentations
with other forms of epithelial disorders.13, 14 Mucin balls seem
to be patient and contact lens dependent. Although the patients
are asymptomatic and no relationship between significant corneal
complications and mucin ball indentations have been observed
or reported, clinical action should be taken for safety purposes
in severe cases with excessive and frequently recurring mucin
balls.
 |
| Figure
8 - click to enlarge |
Summary
The corneal epithelium needs adequate amounts of
oxygen to function optimally and to maintain its normal homeostatic
dynamics. Until
now, contact lenses have not been able to provide the corneal
epithelium with sufficient amounts of oxygen, leading to several
minor and major epithelial changes and complications. Silicone
hydrogel lenses, together with hyper Dk oxygen transmissible
rigid gas permeable lenses, have now virtually eliminated the
problem of hypoxia. This exciting and significant advancement
should certainly benefit the overall health of the corneal epithelium,
even though the elimination of hypoxia may not be the end of
the story. Consequently, a healthy and functional corneal epithelium,
would be expected to be more effective in fighting off infectious
organisms. Does this mean that hyper Dk lenses will entirely
eliminate corneal infection? Unfortunately, the answer is no.
Infections can still occur with silicone hydrogel lenses, albeit
a steep decline in incidence rates is anticipated based on encouraging
clinical data.18 Future epidemiological studies will
reveal the exact incidence rates, nonetheless, careful selection
of EW patients
and adequate clinical monitoring, even with silicone hydrogel
lenses, should still apply in today’s contact lens practice.
Figures
legend
1. Corneal epithelial thickness prior to contact lens wear (0=baseline)
and at several time-points during extended wear. (6N= weekly
replacement; 30N=monthly replacement).3
2. Pseudomonas aeruginosa binding to exfoliated human corneal
epithelial cells (arrow heads).
3. Bacterial binding prior to contact lens wear (0=baseline)
and at several time-points during extended wear.3
4. Corneal epithelial surface cell exfoliation prior to contact
lens wear (0=baseline) and at several time-points during extended
wear.3
5. Number of dead/dying cells on the rabbit corneal epithelium
following short-term EW and eyelid suturing.4
6. Proliferating cells in the rabbit corneal epithelium after
short-term contact lens wear: A. Low Dk RGP, B. hyper Dk RGP,
C. Non-lens wearing control. Bar=50mµm.8
7. Proliferation rate central epithelium in the rabbit cornea
following short-term silicone hydrogel and other types of lens
wear.9
8. A. Severe case of mucin ball related spherical indentations
in the human corneal epithelium as seen by in vivo confocal microscopy.
B. Rabbit model shows distinct holes in the corneal epithelium
with no cell nuclei. (Red staining=cell nuclei)16
|