|
Silicone hydrogel lens materials are unique in the way that they behave. Their extraordinary oxygen transmission [ 1, 2 ] and the physiological benefits that such performance affords these materials is well documented [ 3-7 ]. However, studies have also revealed that there are several other unique characteristics of these materials, particularly in respect to their interaction with the tear film. Compared with conventional hydrogels they dehydrate to a lesser extent and at a slower rate [ 8, 9 ], they adsorb considerably less protein [ 10-13 ], exhibit increased lipid deposition [ 11 ] and display reduced wettability [ 14-16 ] due to the exposure of silicon groups at the material surface [ 17 ]. In view of their unique behaviour, there are growing concerns that their performance warrants them being considered in an FDA grouping that is new and exclusive to such materials [ 18 ].
The recent release of two silicone hydrogel lenses aimed primarily at the daily wear market (Acuvue Advance and O 2 OPTIX) and the reticence of some practitioners to recommend lenses of any type for overnight wear will inevitably result in an increase in the percentage of silicone hydrogels used solely for daily wear. Current estimates indicate that the use of silicone hydrogels for daily wear varies between 10 and 80%, dependent upon the country in question, with an average value of approximately 40% [ 19 ]. Once used in this way, then the issue of which care regimen performs optimally with these lenses becomes a matter of some concern to both practitioner and patient alike. This question becomes particularly relevant, given their unique properties described above.
The major point to consider with regards to solution choice relates to optimizing lens comfort. Up to 50% of contact lens wearers complain of symptoms of dryness and reduced comfort with their lenses [ 20 ], particularly towards the end of the day [ 21 ]. Several studies indicate that patients report fewer symptoms of dryness with silicone hydrogel lenses compared with conventional lenses [ 10, 22-26 ], but it still remains a major reason for dissatisfaction, even with these new materials [ 26, 27 ].
In order to maximize lens comfort and minimise dryness, attempts should be made to enhance the wettability of the lens surfaces - by ensuring that the lens surfaces remain as free of tear deposits as possible - and minimizing any compatibility issues with the solutions used.
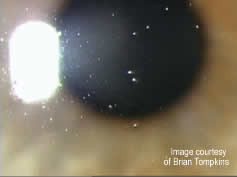
Modern generation care regimens are typically used in a “no-rub” format, where lenses are merely removed from the eye and placed in the regimen overnight, thus optimizing convenience for the patient. However, the majority of current regimens were developed for conventional materials and have been formulated to optimize protein removal, frequently including components such as citric acid and Hydranate® (hydroxyalkylphosphonate) that optimally remove or sequester protein from the lens material or solution. As described above, silicone hydrogels deposit little protein but increased amounts of lipid compared with conventional hydrogel materials. Not infrequently, patients refitted from a group IV hydrogel material such as etafilcon A (Acuvue 2) - which adsorbs very little lipid [ 28, 29 ] - into a silicone hydrogel material will display increased surface “coating” of their lens (Figure 1) and occasionally some evidence of lens calculi or “jelly-bumps” (Figure 2), which are primarily composed of lipid [ 30, 31 ]. The lipid deposition is occurring due to the increased hydrophobicity that exists at the surface of these new lens materials and this lipid contamination will often decrease the wettability of the lens surface and result in symptoms of dryness and visual dissatisfaction, particularly at the end of the day.

|
|
Figure 1 Filmy deposition on a silicone hydrogel lens material, typical of a patient with meibomian gland dysfunction - click to enlarge |
Figure 2 Lens calculi formed on a silicone hydrogel lens material - click to enlarge
|
These patients often display signs of mild blepharitis or meibomian gland dysfunction (MGD) and management of these conditions by using conventional lid hygiene methods will often minimise the lipid deposit on the silicone hydrogel material. In situations where there is no obvious sign of MGD then increased attention to removing the lipid is required. This can be achieved by either using a surfactant cleaner containing alcohol (such as MiraFlow® or AOFlow®) in conjunction with their multipurpose regimen, or using a “no-rub” multipurpose care regimen in a “rubbing” format, where the lens is removed, physically rubbed with the care regimen to aid lipid removal and then soaked as usual overnight. In patients who are primarily using their lenses on an overnight basis, periodic removals and rubbing and rinsing in this manner will often improve lens comfort and overall patient acceptance of their silicone hydrogels.
Compatibility of care regimens with silicone hydrogel materials has recently come under some scrutiny [ 32 ]. The vast majority of modern care regimens used to disinfect contact lenses consist of products that are based upon hydrogen peroxide or two high molecular weight antibacterial agents, namely polyhexamethylene biguanide (PHMB) and polyquaternium-1 (polyquad). The most recent addition to the market-place (Bausch & Lomb ReNu MoistureLoc) uses a novel preservative called Alexidine.
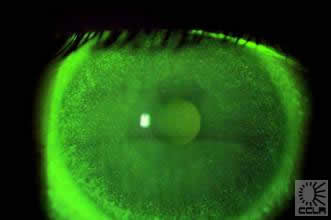
A number of recent studies have suggested that the use of certain care regimens with silicone hydrogels may result in abnormally high levels of relatively asymptomatic corneal staining [ 27, 33-35 ], though other studies have refuted this claim [ 36, 37 ]. While clinical problems associated with the use of “old-type” preservatives and disinfectants are well accepted, complications with newer, high molecular weight preservatives are relatively rare, with millions of patients worldwide successfully using modern, one-step, no-rub, multipurpose products. Studies with silicone hydrogels indicate that the corneal staining seen is frequently annular in appearance (Figure 3), typically increases in severity over a 4-week wearing period [ 27, 35 ], is remarkably asymptomatic [ 27, 35, 38, 39 ] and appears to be at its greatest approximately two hours after insertion, with reduced levels observed six hours after insertion [ 40 ]. This pattern may help to explain why some clinicians and published reports have been relatively unaware of this phenomenon [ 36, 37 ], as practitioners may often see a patient for a follow-up appointment some 6-8 hours post-insertion and may only insert fluorescein if the patient reports any discomfort.
|
Figure 3 Asymptomatic annulus of staining in a patient using a silicone hydrogel with a preserved care product - click to enlarge
|
These observations are of clinical note, as practitioners switching patients from conventional lens materials into daily wear silicone hydrogels will typically allow the patient to continue with their previous care regimen. If a patient were to exhibit a solution-type sensitivity or toxicity of the type described above, it would be logical for the practitioner to blame the lens material, as that is the only factor to have changed. However, it is not the lens material that is at fault but a specific interaction between the material and care regimen that is causing the problem; the same patient may not exhibit this staining pattern if they were to use a different care system that does not react with the silicone hydrogel material.
If a practitioner switches a daily wear patient from a conventional hydrogel material to a silicone hydrogel then the potential for increased corneal staining with some solutions should be considered. A careful fluorescein evaluation, using a yellow barrier filter, must be undertaken at all after-care visits to ensure no undesirable interactions between the lens material and care regimen are occurring and it would appear that examination of patients approximately two hours post lens insertion is most likely to reveal any potential incompatibilities. Subjects exhibiting such reactions should be switched to another regimen and a careful examination of the cornea undertaken some 7-10 days later to ensure that the interaction has been overcome with the new combination.
In conclusion, patients being fitted with daily wear silicone hydrogel lens materials should be carefully followed-up to ensure that an appropriate care regimen has been dispensed. It is likely that manufacturers will, in future, develop care regimens optimised towards silicone hydrogels and that increasingly practitioners will be provided with solution systems specifically formulated for this growing category of lens materials.
References 1. Alvord L, et al.: Oxygen permeability of a new type of high Dk soft contact lens material. Optom Vis Sci 1998 75;1: 30 - 36.
2. Tighe B: Silicone hydrogels: Structure, properties and behaviour. in Silicone Hydrogels: Continuous Wear Contact Lenses, D. Sweeney, Editor. Oxford, Butterworth-Heinemann,2004, pp 1 - 27.
3. Papas EB, et al.: High-oxygen-transmissibility soft contact lenses do not induce limbal hyperaemia. Curr Eye Res 1997 16;9: 942 - 948.
4. du Toit R, et al.: Recovery from hyperemia after overnight wear of low and high transmissibility hydrogel lenses. Curr Eye Res 2001 22;1: 68-73.
5. Dumbleton KA, et al.: Vascular response to extended wear of hydrogel lenses with high and low oxygen permeability. Optom Vis Sci 2001 78;3: 147 - 151.
6. Sweeney DF: Clinical signs of hypoxia with high-Dk soft lens extended wear: is the cornea convinced? Eye Contact Lens 2003 29;1 Suppl: S22-5; discussion S26-9, S192-4.
7. Covey M, et al.: Hypoxic effects on the anterior eye of high-Dk soft contact lens wearers are negligible. Optom Vis Sci 2001 78;2: 95-99.
8. Jones L, et al.: In vitro evaluation of the dehydration characteristics of silicone-hydrogel and conventional hydrogel contact lens materials. Contact Lens & Ant Eye 2002 25; 147 - 156.
9. Morgan PB, Efron N: In vivo dehydration of silicone hydrogel contact lenses. Eye Contact Lens 2003 29;3: 173-176.
10. McNally J, McKenney CD: A clinical look at a silicone hydrogel extended wear lens. Contact Lens Spectrum 2002 17;1: 38 - 41.
11. Jones L, et al.: Lysozyme and lipid deposition on silicone hydrogel contact lens materials. Eye Contact Lens 2003 29;1 Suppl: S75-S79.
12. Senchyna M, et al.: Quantitative and conformational characterization of lysozyme deposited on balafilcon and etafilcon contact lens materials. Curr Eye Res 2004 28;1: 25-36.
13. Subbaraman LN, et al.: Stabilization of lysozyme mass extracted from lotrafilcon silicone hydrogel contact lenses. Optom Vis Sci 2005 82;3: 209-14.
14. Bruinsma GM, van der Mei HC, Busscher HJ: Bacterial adhesion to surface hydrophilic and hydrophobic contact lenses. Biomaterials 2001 22;24: 3217-24.
15. Court JL, et al.: A novel phosphorylcholine-coated contact lens for extended wear use. Biomaterials 2001 22;24: 3261-72.
16. Cheng L, Muller SJ, Radke CJ: Wettability of silicone-hydrogel contact lenses in the presence of tear-film components. Curr Eye Res 2004 28;2: 93-108.
17. Karlgard C, et al.: Drying methods for XPS analysis of PureVision, Focus Night&Day and conventional hydrogel contact lenses. Appl Surface Sci 2004 230; 106 - 114.
18. Sczotke-Flynn L: Advocating a new lens group. Contact Lens Spectrum 2005 20;2: 23.
19. Morgan P, et al.: International contact lens prescribing in 2004. Contact Lens Spectrum 2005 20;1: 34 - 37.
20. Doughty MJ, et al.: A patient questionnaire approach to estimating the prevalence of dry eye symptoms in patients presenting to optometric practices across Canada. Optom Vis Sci 1997 74;8: 624-31.
21. Fonn D, Dumbleton K: Dryness and discomfort with silicone hydrogel contact lenses. Eye Contact Lens 2003 29;1 Suppl: S101-4; discussion S115-8, S192-4.
22. Sweeney D, et al.: Clinical performance of silicone hydrogel lenses. in Silicone hydrogels: Continuous wear contact lenses, D. Sweeney, Editor. Oxford, Butterworth-Heinemann,2004, pp 164 - 216.
23. Aakre BM, et al.: A 6-month follow-up of successful refits from daily disposable soft contact lenses to continuous wear of high-Dk silicone-hydrogel lenses. Ophthalmic Physiol Opt 2004 24;2: 130-41.
24. Sickenberger W, et al.: Are Fl-Si-Hydrogel-CL suitable for dry eye conditions in daily wear mode? AAO International Meeting. 2002.
25. Chalmers R, et al.: The role of dryness symptoms in discontinuation of wear and unscheduled lens removals with extended wear of silicone hydrogel materials. ARVO, Abstract # 3088. 2002.
26. Fonn D, Pritchard N, Dumbleton K: Factors affecting the success of silicone hydrogels. in Silicone Hydrogels: The Rebirth of Continuous Wear Contact Lenses, D. Sweeney, Editor. Oxford, UK, Butterworth-Heinemann,2000, pp 214 - 234.
27. Jones L, MacDougall N, Sorbara LG: Asymptomatic corneal staining associated with the use of balafilcon silicone-hydrogel contact lenses disinfected with a polyaminopropyl biguanide-preserved care regimen. Optom Vis Sci 2002 79;12: 753 - 761.
28. Jones L, et al.: An in vivo comparison of the kinetics of protein and lipid deposition on group II and group IV frequent-replacement contact lenses. Optom Vis Sci 2000 77;10: 503-10.
29. Maissa C, et al.: Influence of contact lens material surface characteristics and replacement frequency on protein and lipid deposition. Optom Vis Sci 1998 75;9: 697-705.
30. Hart DE, Tidsale RR, Sack RA: Origin and composition of lipid deposits on soft contact lenses. Ophthalmology 1986 93;4: 495-503.
31. Bowers R, Tighe B: Studies of the ocular compatibility of hydrogels. White spot deposits - chemical composition and geological arrangement of components. Biomaterials 1987 8; 172-177.
32. Jones L: Understanding incompatibilities. Contact Lens Spectrum 2004 19;7 (suppl): 4 - 7.
33. Epstein A: SPK with daily wear of silicone hydrogel lenses and MPS. Contact Lens Spectrum 2002 17;11: 30.
34. Fonn D: Observations of corneal staining with MPS and silicone hydrogel lenses. Contact Lens Spectrum 2002 17;11: 32.
35. Jones L, et al.: Clinical performance and corneal staining associated with silicone hydrogels used on a daily wear basis. Abstract - AAO Meeting, Hawaii, Apr 2004 2004.
36. DePaolis M, Ryan R: Silicone hydrogel lenses in practice. Contact Lens Spectrum 2002 17;11: 36 - 38.
37. Levy B: Contact lens and solution manufacturer responds with data. Contact Lens Spectrum 2002 17;11: 34.
38. Amos C: Performance of a new multipurpose solution used with silicone hydrogels. Optician 2004 227;5945: 18-22.
39. Amos C: A clinical comparison of two soft lens care systems used with silicone hydrogel contact lenses. Optician 2004 227;5933: 16 - 20.
40. Garofalo R, Dassanayake N: Corneal response of chemical agents released by hydrogel and silicone hydrogel lenses as a function of time. ARVO abstracts 2004 e-abstract # 1538.
|