In 1999, two silicone hydrogel contact lenses – Bausch & Lomb PureVision and CIBA Vision Focus Night & Day – were launched in many markets around the world. Both lens types were almost exclusively marketed for extended wear and the two companies initiated significant educational programs for practitioners, many of whom had little or no experience of prescribing contact lenses for overnight wear.
As reported on this website [1], the uptake of this new modality by contact lens practitioners was generally positive, albeit with significant differences between countries. Importantly, the prescribing of extended wear silicone hydrogels was typically two-times greater to existing contact lens wearers compared to new wearers. This difference demonstrated that contact lens practitioners were much more likely to recommend this new modality of contact lens wear to patients for whom some clinical history was readily available (including information about previous adverse events, compliance etc.) than to new contact lens wearers.
Early experiences with the new lens type for extended wear were typically good with clinical findings matching research experience in the reduction or elimination of signs of hypoxia such as corneal epithelial microcysts [2]. At the same time, increased levels of clinically significant papillary conjunctivitis (3% of eyes per year [3]) and superior epithelial arcuate lesions (also 3% of eyes per year [3]) were less favourable findings. The number of inflammatory responses has also been reported as being higher with extended wear silicone hydrogel lenses than with daily wear contact lenses. Although reports of the incidence of such events vary considerably, it might be as high as 15% of wearers per year [4,5]. More recently, the incidence of inflammatory events requiring hospital management has been reported as being much higher with silicone hydrogel extended wear lenses than with daily wear soft lenses [6], although the severity of these events appears to be reduced in silicone hydrogel extended wear lenses compared with the extended wear of conventional hydrogels [6]. Stapleton and colleagues have recently reported increased incidence of microbial keratitis in silicone hydrogel extended wear compared with conventional hydrogel daily wear, and no difference between the two forms of extended contact lens wear – silicone hydrogel vs. conventional hydrogel [7].
The above assessment suggests that whilst extended wear silicone hydrogel contact lenses offer a range of benefits to the wearer in terms of convenience and cosmesis, there are a number of clinical challenges which remain to be overcome. These, in turn, may have contributed to the plateau which is now seen in the prescribing of extended wear contact lenses (Figure 1). In countries which initially saw strong interest in extended wear, such as Australia and Canada, there now appears to be a modest decline. A similar pattern can be seen in the United Kingdom although continued growth with this modality is apparent in Norway where extended wear lenses account for 24% of soft lens fits.
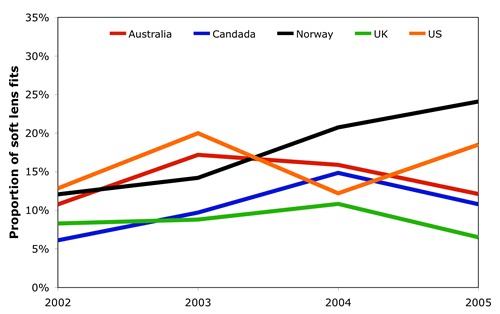 |
| Figure 1 - The proportion of soft lenses prescribed on an extended wear basis in five countries. In the United States, a significant proportion of extended wear lenses are conventional (non silicone hydrogel) hydrogels. In the other four countries shown, almost all the extended wear lenses are silicone hydrogels. |
An important development in the introduction of silicone hydrogel materials into the global market was the launch of the first silicone hydrogel lens specifically marketed for daily wear – Johnson & Johnson Acuvue Advance – which was introduced in early 2004 and which was followed by two further products – CIBA Vision O2 Optix and Johnson & Johnson Acuvue Oasys. With the re-positioning of Bausch & Lomb PureVision as also a daily wear product in some markets, contact lens practitioners now have a range of silicone hydrogel lenses to prescribe for daily wear. These new lenses typically meet the requirements of Papas [8] (peripheral Dk/t of at least 56 units) for inducing no limbal hyperaemia, a finding which has been confirmed clinically by Maldonado-Codina and colleagues [9]. Due to the recent introduction of the daily wear silicone hydrogel modality, there are little clinical data available in the literature, but on the basis that most of the adverse events seen with extended wear silicone hydrogel lenses are principally related to overnight wear, practitioners should expect to see signs of improved oxygen delivery to the ocular surface [10] with very few adverse events.
Early evidence from the market has seen daily wear silicone hydrogel prescribing quickly exceed that for extended wear silicone hydrogels after only about one year of products specifically marketed for daily wear. Figure 2 shows the proportion of silicone hydrogels prescribed for daily wear. In four of the five major markets shown, more daily wear silicone hydrogels were prescribed than extended wear silicone hydrogels. A good example here is Canada. In early 2004, 8% of daily wear soft lens fits were with silicone hydrogel materials. By early 2005, this was 33%. Whilst it remains to be seen if this large increase in usage will be maintained, early evidence from the market suggest that there is a clear acceptance by contact lens practitioners around the world of silicone hydrogel materials for daily wear, whist their use for extended wear appears to have hit a glass ceiling.
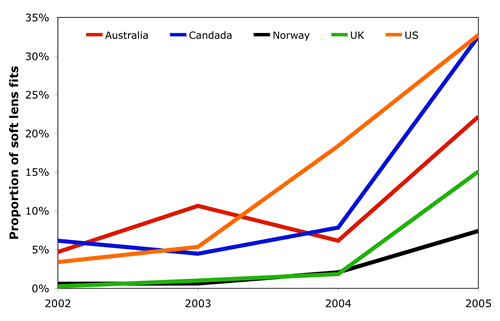 |
| Figure 2 - The proportion of silicone hydrogel lenses prescribed for soft lens daily wear |
Future trends
One model for future prescribing patterns may see the daily wear soft lens market become dominated by daily disposable conventional lenses and silicone hydrogel planned replacement (2-weekly or monthly) lenses. Given the strong moves in the market place towards silicone hydrogel daily wear, and the manufacturing/logistical implications precluding silicone hydrogel daily disposables in the short term, this scenario seems quite possible over the next two or three years. At the same time, extended wear silicone hydrogels could see further growth as newer materials seek to overcome the current clinical challenges of this modality. Some of the inflammatory responses seen with these lenses appear to have a mechanical aetiololgy [11] and the softer, lower-modulus materials becoming available may see a reduction in this type of adverse event. At the same time, these materials could reduce the incidence of other mechanically-mediated clinical problems such as papillary conjunctivitis and superior epithelial arcuate lesions. In addition, the much-discussed advent of anti-microbial surfaces potentially offer reduced levels of contact lens associated infection and inflammation.
More information on current prescribing trends is published in the January 2006 issue of Contact Lens Spectrum.
References
- http://www.siliconehydrogels.org Editorial, December 2004.
- Keay L, Sweeney DF, Jalbert I, Skotnitsky C and Holden BA. Microcyst response to high Dk/t silicone hydrogel contact lenses. Optom Vis Sci 2000; 77: 582-5.
- Dumbleton K. Noninflammatory silicone hydrogel contact lens complications. Eye Contact Lens 2003; 29 (1s): s186-9.
- Dumbleton K. Adverse events with silicone hydrogel continuous wear. Contact Lens Ant Eye 2002; 25: 137-46.
- Morgan PB, Efron N, Maldonado-Codina C, Efron S. Adverse events and discontinuations with rigid and soft hyper Dk contact lenses used for continuous wear. Optom Vis Sci 2005; 82: 528-35.
- Efron N, Morgan PB, Hill EA, Raynor MK, Tullo AB. Incidence and morbidity of hospital-presenting corneal infiltrative events associated with contact lens wear. Clin Exp Optom 2005; 88: 232-239.
- Stapleton F, Edwards K, Keay L, et al. The Incidence of contact lens associated microbial keratitis in Australia. ARVO Abstracts 2005;46:E-abstract 5025.
- Papas E. On the relationship between soft contact lens oxygen transmissibility and induced limbal hyperaemia. Exp Eye Res 1998; 67: 125-31.
- Maldonado-Codina C, Morgan PB, Schnider CM, Efron N. Short-term physiologic response in neophyte subjects fitted with hydrogel and silicone hydrogel contact lenses. Optom Vis Sci 2004; 81: 911-921.
- Brennan NA. Beyond flux: total corneal oxygen consumption as an index of corneal oxygenation during contact lens wear. Optom Vis Sci 2005; 82: 467-72.
- Efron N, Morgan PB, Hill EA, Raynor MK, Tullo AB. The size, location and clinical severity of corneal infiltrative events associated with contact lens wear. Optom Vis Sci 2005; 82: 519-527.
|