The classification, incidence, and prevalence of adverse events associated with silicone hydrogel contact lens wear depends on how one defines an adverse event. What qualifies as an adverse event? By some Institutional Review Board definitions, an adverse event can range from any condition that disrupts routine daily living activities without major discomfort and interferences that do not persist, to death. To that end, an asymptomatic clinician-observed slit lamp sign that necessitates temporary discontinuation of lens wear would be classified as an adverse event. Therefore, one may ask, has the incidence of unwelcome biomicroscopy signs such as neovascularization, epithelial microcysts, stromal edema, and polymegathism decreased since the introduction of silicone hydrogel lenses? Alternatively, once may ask the question whether traditionally defined adverse events have declined with silicone hydrogel lens use. These contact lens associated adverse events include symptomatic ocular surface changes such as infection, inflammation, and mechanically induced complications. Most of the adverse findings reported in the literature are secondary to extended wear contact lens use, therefore, I will concentrate on these findings unless otherwise mentioned.
In response to the first question: “Have adverse biomicroscopy findings declined with silicone hydrogel contact lens use?”; the answer is almost an unanimous “YES”! The clinical performance of silicone hydrogel lenses clearly demonstrates an improvement compared to low Dk lenses on a number of physiological markers making them especially desirable for continuous wear. Limbal
 |
click to enlarge |
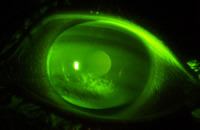
| Figure 1, central corneal staining observed with silicone hydrogel lens wear |
hyperemia is not induced or, if present, is significantly reduced with silicone hydrogel lens use.[1] Limbal neovascular vessels induced from traditional hydrogels empty after refitting with silicone hydrogels.[2] There is no increased epithelial microcyst response.[3] No endothelial cell stress is observed [4] compared to studies of traditional hydrogels that show up to 22% increases in endothelial polymegathism.[5, 6] However, with daily wear use a new dilemma has presented itself secondary to unanticipated interactions of some silicone hydrogel lens materials and preserved multi-purpose solutions. Unacceptable levels of asymptomatic corneal staining can occur which necessitates alteration of the lens-solution combination.[7](Figure 1) As of yet, asymptomatic corneal staining has not been universally considered as an adverse event, but if lens wear is discontinued for a period of time to manage it, one can think of it as a type of an adverse response. Recently, two studies have associated corneal staining as a risk factor for corneal infiltrates[8, 9], thus more attention may now be paid to this biomicroscopy finding by clinicians and researchers alike.
In response to the second question: “Have infections, inflammation, and mechanical events declined with silicone hydrogel lens use?”; the answers are not as clear nor are they as positive. Case reports of microbial keratitis with silicone hydrogel lenses are still being reported in the literature.[10-13] Corneal infiltrates continue to occur, and contact lens papillary conjunctivitis (CLPC) continues to be present in different forms, time points, and rates compared to traditional hydrogel extended wear.[14, 15]
CLPC is one of the leading causes of patient discontinuation from contact lens wear. General CLPC involves raised papillae with moderate to severe hyperemia across the entire tarsus. General CLPC
is characterized by moderate to severe patient symptoms, including itching or irritation, a stringy or
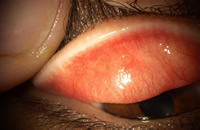
ropy discharge, excessive movement of the lens and blurred vision secondary to lens movement or coatings. The time to occurrence in silicone hydrogel lens wear is on average 11 months but ranges from 6-17 months.[14] In silicone hydrogel lens wear a greater proportion of patients develop a localized form of CLPC, such as that seen with exposed sutures, which is more easily treatable by discontinuing lens wear.[14] (Figure 2)
 |
click to enlarge |
Figure 2, localized CLPC after
one month continuous wear of a silicone hydrogel lens in a neophyte patient |
This form of CLPC has been proposed to be mechanical in nature from a less than ideal relationship between the lens edge and lid surface, from the higher modulus of elasticity of the lens material, or from differences in ocular surface topography creating poor lens drapage and fitting characteristics. The time to occurrence varies considerably, sometimes a few weeks after commencing silicone hydrogel lens wear, but in other cases not until at least two years of lens wear. In the lotrafilcon A, balafilcon A, and senofilcon A pre-market approval studies in the U.S. the annual incidence of tarsal abnormalities in the control etafilcon A arm ranged from 0.1-3.9%, and in the silicone hydrogel study lens arm ranged from 0.7 - 3.9%.
Superior Epithelial Arcuate Lesions (SEALs) are thin, arcuate white lesions within 1-3 mm of the superior limbus usually between 10-2 o’clock in an area normally covered by the upper eyelid. The SEAL has significant overlying staining and possible underlying diffuse infiltrates. The most common symptom is foreign body sensation or irritation. SEALs are thought to be caused by mechanical stress on the superior cornea from mechanical chaffing and rubbing of the cornea from stiffer lens materials under the upper lid and were hypothesized to increase when silicone hydrogel lenses were released.[16] In the lotrafilcon A pre-market approval study there were no SEALS in the etafilcon A control arm and 0.15% in the 30 day silicone hydrogel arm.
Microbial keratitis (MK)
In 1989 the U.S. Food and Drug Administration (FDA) reverted 30 day continuous wear approvals to 7 day extended wear for lenses made of low oxygen permeable (Dk) materials secondary to published reports that the risk of ulcerative keratitis was significantly higher when lenses were worn overnight.[17, 18] Schein et al[17] reported overnight users of low Dk lenses had a 10-15X greater risk of ulcerative keratitis compared to daily wearers, and Poggio et al[18] reported the annualized incidence of ulcerative keratitis was 20.9 per 10,000 lens wearers when worn for 7 days extended wear. Other studies confirm these rates for low Dk materials.[19, 20]
One of the early epidemiological studies to assess the impact of silicone hydrogel lens use compared to low Dk lenses on the risk of severe keratitis, suggested about a 5-fold decline in the silicone hydrogel group. [21] However, other studies to assess the risk of MK with silicone hydrogel lenses [22] documented the annual rate was 18 per 10,000 wearers for a 30 night CW schedule, similar to that reported for low Dk conventional and disposable extended wear lenses worn for fewer consecutive nights. Other groups have reported similar findings.[23] One study found the overall incidence of contact lens-related keratitis which required hospital treatment was about the same for silicone hydrogel and low Dk extended wear, however, the clinical severity of the keratitis was lower in the silicone hydrogel group compared with the low Dk group.[21, 24] Therefore, the general consensus is that silicone hydrogel lenses when worn for up to 30 days continuous wear do not reduce the risk of MK compared to low Dk lenses when worn for approximately 7 days of wear.
Corneal Infiltrates
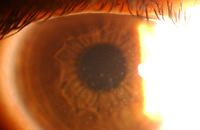
Corneal inflammatory events are a result of a stimulus that has caused directed infiltration of leukocytes into the cornea.[25] (Figure 3) Two studies have assessed the prevalence and/or incidence of corneal infiltrates in non-contact lens wearers.[26, 27] Hickson reported the prevalence of subepithelial microinfiltrates to be 4% of non-contact lens wearing patients[26] and Sankaridurg reported 1.6% of eyes had asymptomatic infiltrates at baseline of a longitudinal study enrolling non-contact lens wearing myopes in India.[27] Nonetheless, it is well documented that extended wear use of contact lenses significantly increases the risk.
 |
click to enlarge |
| Figure 3, infiltrative keratitis in a silicone hydrogel wearer |
Reports of corneal infiltrates with silicone hydrogel lenses reported around the globe vary greatly depending on the definition and study design used to capture the infiltrative event. The incidence proportions range from 2% to over 21%.[28-35] A recent meta-analysis determined that the risk of corneal infiltrates is approximately two-fold higher in SH lenses when worn for 30 days continuous wear compared to their low Dk counterparts when worn for 7 days continuous wear.[36] In this meta-analysis the rates of infiltrates for low Dk hydrogels and silicone hydrogel lenses were 7.7 (2.2, 26.7) and 14.4 (4.3, 48.2) per 100 eye-years, respectively. However, it should be pointed out that the increased risk cannot be definitively linked to the effect of lens material because the effect of material on outcome is confounded by length of wear.
Summary
Most published studies of silicone hydrogel lens related adverse events include patients wearing lenses for up to 30 days continuous wear, and low Dk lenses for up to 7 days of wear. The rates of hypoxic related problems are significantly decreased with silicone hydrogel lens use. The risk of microbial keratitis is unchanged, but the severity of the infection may be lower with silicone hydrogel lens use. Rates of mechanical events may be unchanged or slightly higher in silicone hydrogel lenses, and the appearance of CLPC differs in silicone hydrogel lens wear. Sterile infiltrates have approximately doubled compared to low Dk lenses. It is hypothesized by some that a 30 day wear schedule with silicone hydrogel lenses may predispose the patient to some of these adverse events more than the material. Based on the vast ocular physiological improvements documented with highly oxygen transmissible silicone hydrogel materials, the quick recovery of mechanical problems, the relatively low severity of corneal inflammatory events, and the fact that many of the sterile corneal infiltrates are asymptomatic and possibly a normal ocular response to the environment, I continue to promote silicone hydrogel lenses as the lens of choice for extended wear and daily wear. However, new generation materials and perhaps a shortened length of wear should be investigated to continue to reduce adverse events to lower levels than that seen with low Dk materials.
- Papas, E.B., C.M. Vajdic, R. Austen, and B.A. Holden, High-Oxygen-Transmissibility Soft Contact Lenses Do Not Induce Limbal Hyperaemia. Curr Eye Res, 1997. 16(9): p. 942-8.
- Dumbleton, K., Richter D, Simpson, Fonn D., A Comparison of the Vascular Response to Extended Wear of Conventional Lower Dk and Experimental High Dk Hydrogel Contact Lenses. Optom Vis Sci, 1998. 75(12s): p. 170.
- Keay, L., D.F. Sweeney, I. Jalbert, C. Skotnitsky, and B.A. Holden, Microcyst Response to High Dk/T Silicone Hydrogel Contact Lenses. Optom Vis Sci, 2000. 77(11): p. 582-5.
- Doughty, M.J., B.M. Aakre, A.E. Ystenaes, and E. Svarverud, Short-Term Adaptation of the Human Corneal Endothelium to Continuous Wear of Silicone Hydrogel (Lotrafilcon a) Contact Lenses after Daily Hydrogel Lens Wear. Optom Vis Sci, 2005. 82(6): p. 473-80.
- Holden, B.A., D.F. Sweeney, A. Vannas, K.T. Nilsson, and N. Efron, Effects of Long-Term Extended Contact Lens Wear on the Human Cornea. Invest Ophthalmol Vis Sci, 1985. 26(11): p. 1489-501.
- Holden, B.A., A. Vannas, K. Nilsson, et al., Epithelial and Endothelial Effects from the Extended Wear of Contact Lenses. Curr Eye Res, 1985. 4(6): p. 739-42.
- Jones, L., N. MacDougall, and L.G. Sorbara, Asymptomatic Corneal Staining Associated with the Use of Balafilcon Silicone-Hydrogel Contact Lenses Disinfected with a Polyaminopropyl Biguanide-Preserved Care Regimen. Optom Vis Sci, 2002. 79(12): p. 753-61.
- Carnt, N., I. Jalbert, T. Naduvilath, and E. Papas, Solution Toxicity in Soft Contact Lens Daily Wear Is Associated with Corneal Inflammation. Opt Vis Sci, IN PRESS.
- Szczotka-Flynn, L., S.M. Debanne, V.K. Cheruvu, et al., Predictive Factors for Corneal Infiltrates with Continuous Wear of Silicone Hydrogel Contact Lenses. Arch Ophthalmol, 2007. 125(4): p. 488-92.
- Holden, B.A., D.F. Sweeney, P.R. Sankaridurg, et al., Microbial Keratitis and Vision Loss with Contact Lenses. Eye Contact Lens, 2003. 29(1 Suppl): p. S131-4; discussion S143-4, S192-4.
- Lee, K.Y. and L. Lim, Pseudomonas Keratitis Associated with Continuous Wear Silicone-Hydrogel Soft Contact Lens: A Case Report. Eye Contact Lens, 2003. 29(4): p. 255-7.
- Syam, P., B. Hussain, and C. Hutchinson, Mixed Infection (Pseudomonas and Coagulase Negative Staphylococci) Microbial Keratitis Associated with Extended Wear Silicone Hydrogel Contact Lens. Br J Ophthalmol, 2004. 88(4): p. 579.
- Whiting, M.A., M.K. Raynor, P.B. Morgan, et al., Continuous Wear Silicone Hydrogel Contact Lenses and Microbial Keratitis. Eye, 2004. 18(9): p. 935-7.
- Skotnitsky, C., P.R. Sankaridurg, D.F. Sweeney, and B.A. Holden, General and Local Contact Lens Induced Papillary Conjunctivitis (Clpc). Clin Exp Optom, 2002. 85(3): p. 193-7.
- Skotnitsky, C.C., T.J. Naduvilath, D.F. Sweeney, and P.R. Sankaridurg, Two Presentations of Contact Lens-Induced Papillary Conjunctivitis (Clpc) in Hydrogel Lens Wear: Local and General. Optom Vis Sci, 2006. 83(1): p. 27-36.
- Holden, B.A., A. Stephenson, S. Stretton, et al., Superior Epithelial Arcuate Lesions with Soft Contact Lens Wear. Optom Vis Sci, 2001. 78(1): p. 9-12.
- Schein, O.D., R.J. Glynn, E.C. Poggio, J.M. Seddon, and K.R. Kenyon, The Relative Risk of Ulcerative Keratitis among Users of Daily-Wear and Extended-Wear Soft Contact Lenses. A Case-Control Study. Microbial Keratitis Study Group. N Engl J Med, 1989. 321(12): p. 773-8.
- Poggio, E.C., R.J. Glynn, O.D. Schein, et al., The Incidence of Ulcerative Keratitis among Users of Daily-Wear and Extended-Wear Soft Contact Lenses. N Engl J Med, 1989. 321(12): p. 779-83.
- Cheng, K.H., S.L. Leung, H.W. Hoekman, et al., Incidence of Contact-Lens-Associated Microbial Keratitis and Its Related Morbidity. Lancet, 1999. 354(9174): p. 181-5.
- Nilsson, S.E. and P.G. Montan, The Annualized Incidence of Contact Lens Induced Keratitis in Sweden and Its Relation to Lens Type and Wear Schedule: Results of a 3-Month Prospective Study. Clao J, 1994. 20(4): p. 225-30.
- Morgan, P.B., N. Efron, E.A. Hill, et al., Incidence of Keratitis of Varying Severity among Contact Lens Wearers. Br J Ophthalmol, 2005. 89(4): p. 430-6.
- Schein, O.D., J.J. McNally, J. Katz, et al., The Incidence of Microbial Keratitis among Wearers of a 30-Day Silicone Hydrogel Extended-Wear Contact Lens. Ophthalmology, 2005. 112(12): p. 2172-9.
- Stapleton F, K.L., Edwards K, Naduvilath T, Dart J, Brian G, Holden B., Risks of Contact Lens Related Microbial Keratitis. Optom Vis Sci, 2005. 82(E-abstract 050068.).
- Efron, N., P.B. Morgan, E.A. Hill, M.K. Raynor, and A.B. Tullo, Incidence and Morbidity of Hospital-Presenting Corneal Infiltrative Events Associated with Contact Lens Wear. Clin Exp Optom, 2005. 88(4): p. 232-9.
- Josephson, J.a.C., B., Infiltrative Keratitis in Hydrogel Lens Wearers. Int Contact Lens Clin, 1979. 6: p. 223-241.
- Hickson, S. and E. Papas, Prevalence of Idiopathic Corneal Anomalies in a Non Contact Lens-Wearing Population. Optom Vis Sci, 1997. 74(5): p. 293-7.
- Sankaridurg, P.R., D.F. Sweeney, B.A. Holden, et al., Comparison of Adverse Events with Daily Disposable Hydrogels and Spectacle Wear: Results from a 12-Month Prospective Clinical Trial. Ophthalmology, 2003. 110(12): p. 2327-34.
- Aakre, B.M., A.E. Ystenaes, M.J. Doughty, et al., A 6-Month Follow-up of Successful Refits from Daily Disposable Soft Contact Lenses to Continuous Wear of High-Dk Silicone-Hydrogel Lenses. Ophthalmic Physiol Opt, 2004. 24(2): p. 130-41.
- Bausch&Lomb, Fda Summary of Safety and Effectiveness Data for a Supplemtal Premarket Application for Balafilcon a Hydrophilic Contact Lenses. 2001.
- Brennan, N.A., M.L. Coles, T.L. Comstock, and B. Levy, A 1-Year Prospective Clinical Trial of Balafilcon a (Purevision) Silicone-Hydrogel Contact Lenses Used on a 30-Day Continuous Wear Schedule. Ophthalmology, 2002. 109(6): p. 1172-7.
- Ciba, V.C., Summary of Safety and Effectiveness Data for 30 Day Wear of Lotrafilcon A. 2001.
- Dumbleton K, F.D., Jones L, Williams-Lyn, Richter D., Severity and Management of Contact Lens Related Complications with Continuous Wear of High Dk Silicone Hydrogel Lenses. Optometry and Vision Science, 2000. 17(12s): p. XX.
- Fonn, D., K.E. MacDonald, D. Richter, and N. Pritchard, The Ocular Response to Extended Wear of a High Dk Silicone Hydrogel Contact Lens. Clin Exp Optom, 2002. 85(3): p. 176-82.
- Nilsson, S.E., Seven-Day Extended Wear and 30-Day Continuous Wear of High Oxygen Transmissibility Soft Silicone Hydrogel Contact Lenses: A Randomized 1-Year Study of 504 Patients. Clao J, 2001. 27(3): p. 125-136.
- Vistakon, Fda Summary of Safety and Effectiveness Data for a Supplemental Premarket Application for Senofilcon a Hydrophilic Contact Lenses. www.fda.gov/cdrh/pdf4/k042275.pdf, 2005.
- Szczotka-Flynn, L. and M. Diaz-Insua, Risk of Corneal Inflammatory Events with Silicone Hydrogel and Low Dk Hydrogel Extended Contact Lens Wear: A Meta-Analysis. Optometry & Vision Science, 2007. 125: p. 1-5.
|