In 2007, two new lens types were introduced into the silicone hydrogel contact lens market, Biofinity (comfilcon A, CooperVision, Inc) and PremiO (asmofilcon A, Menicon). More recently Avaira (enfilcon A, CooperVision, Inc) has been released. These are the first entries onto the silicone hydrogel market for Menicon, a company synonymous with high oxygen gas permeable lenses, and CooperVision, whose Proclear soft disposable product continues to do well in an ever increasing silicone hydrogel daily wear market.
Lens parameters of the new generation lenses are detailed in Table 1.
Table 1. Lens parameters of silicone hydrogel lenses introduced since 2007 |
|
Biofinity ® |
PremiO |
Avaira™ |
Manufacturer |
CooperVision |
Menicon |
CooperVision |
Base Curve (mm) |
8.6 |
8.6 / 8.3 |
8.5 |
Lens Diameter (mm) |
14 |
14 |
14.2 |
Centre thickness
@ -3.00D (mm) |
0.08 |
0.08 |
0.08 |
Back vertex power (D) |
-0.25 to -10.00 |
+6.00 to -13.00 |
-0.25 to -6.00 |
Surface treatment |
None |
Yes (Nanogloss™) |
None |
Indications |
Daily and up to 1 month extended wear* |
Daily and up to 1 week extended wear |
Daily wear |
Recommended replacement schedule |
4 weeks |
2 weeks daily and 1 week extended wear |
2 weeks |
FDA Group |
1 |
1 |
1 |
| *Daily wear in US and continuous wear in UK and Australia/NZ |
WHAT IS DIFFERENT ABOUT THE 3RD GENERATION LENSES?
The pioneering first generation lenses, PUREVISION™ Vision (balafilcon A, Bausch & Lomb) and NIGHT & DAY™ (lotrafilcon A, CIBA VISION), were introduced into the contact lens market almost 10 years ago and continue to perform well. They utilise phase separation of silicone and hydrogel monomers in their polymer composition. [1] Plasma surface treatments, in the form of plasma coating for NIGHT & DAY™ and plasma oxidation for PUREVISION™, [1] are required to provide wettability. These lenses have high oxygen transmissibility (Dk), low water content and relatively high lens modulus or “stiffness”.
In 2004, we saw the release of intrinsically wettable silicone hydrogel lens materials, such as ACUVUEÒ ADVANCE™ (galyfilcon A, Johnson & Johnson) and ACUVUEÒ OASYS™ (senofilcon A, Johnson & Johnson). These lenses, which are a combination of monomers and macromers, incorporate an internal wetting agent, polyvinyl pyrrolidone (PVP) and, thus, require no surface treatment. With these lenses we see a higher Dk with higher water content than would be expected with first generation lenses, and correspondingly lower modulus, related to the higher water content.
With the 3rd generation, a unique group of materials present themselves, despite their differences. For Biofinity, macromers are the only source of silicone and no surface treatment or internal wetting agent is required. PremiO combines siloxane and hydrophilic monomers using a patented polymerization system (MeniSilk™). A plasma surface treatment (Nanogloss™) renders the surface of PremiO lenses smooth with a low contact angle of 27° (captive bubble method, Menicon data on file). These lenses still show a decrease in modulus with higher water content, but break the traditional inverse relationship between Dk and water content by having a higher Dk than water content predicts as can be seen in Figure 1.
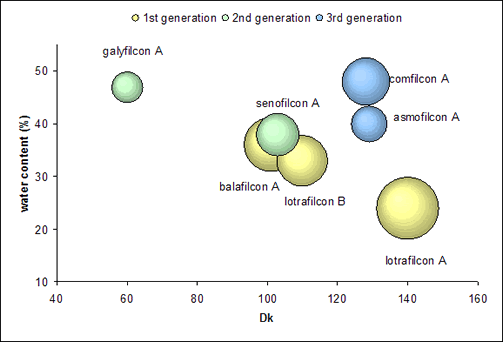 |
Figure 1. The relationship between Dk, water content and modulus (modulus is depicted by the size of the bubble) |
HOW DO THEY PERFORM?
As yet, no information is readily available on the performance of Avaira. A prototype PremiO lens (with a different surface treatment to the commercial product) has been reported as similar in comfort and fitting for the 8.6 and 8.3 base curves, and compared to senofilcon A in short term contralateral evaluation [2]. Biofinity has been shown to perform well in overnight swelling studies [3], showing no difference compared to lotrafilcon A after 8 hours overnight wear in 20 neophyte wearers at CCLR in Canada. A 12 month prospective clinical 30 night contralateral extended wear trial in Australia by Noel Brennan’s group showed better performance for Biofinity for some comfort scores compared to lotrafilcon A and balafilcon A, and some vision variables, objective redness and conjunctival staining compared to lotrafilcon A. [4]
WHAT IS THE PROMISE?
Less Corneal and Conjunctival Interactions?
Traditionally we have seen adverse events likely to be at least in part mechanically induced in silicone hydrogel extended wear, such as superior epithelial arcuate lesions, [5] corneal erosions [6] and contact lens papillary conjunctivitis. [7] Lower modulus, among other factors, such as lens design and diameter, is likely to decrease the incidence of these events in daily and extended wear, without sacrificing the paramount benefits of increased oxygen.
Biofinity and Avaira have rounded edge designs, while PremiO has a “thin and semi-round edge” according to the manufacturers. One of the challenges with silicone hydrogel cast moulded lenses is to achieve complete closure of the mould and not allow any leakage of material at the edges. Edge and lens quality is paramount, which may present difficulties with new technologies.
Biofinity and Avaira do not have surface coating or intrinsic wetting agent. PremiO has Nanogloss technology. Smooth surfaces are likely to decrease friction on the cornea and conjunctiva. How these materials perform in terms of lens deposition, particularly lipid that silicone hydrogel lenses are prone to, will be interesting, particularly with recent correlations between laboratory analysis and clinical findings. [8]
Solution induced corneal staining (SICS) has been shown to vary with different lens types and solutions. [9,10] While Biofinity [9] and PremiO [11] lenses perform well in short-term testing, longer term evaluation of Biofinity, PremiO and Avaira is essential as SICS has been associated with a 3-fold increase in low grade corneal inflammation [12] and decreased comfort. [10]
Better Vision?
Biofinity has aspheric front surface optics and PremiO spherical optics. While Biofinity performed better than lotrafilocn A for subjective vision in a contralateral extended wear trial, [4] preliminary data suggests that different power profiles may not show clinical differences in short term evaluation [13]. It is likely that other factors such as lens surface and lens/corneal alignment play a role in subjective and objective visual acuity.
Better Comfort?
Lens surface, lens corneal alignment, edge profile and quality, interactions with lens solutions are all probable factors to be considered in producing a lens with optimum comfort.
What is clear is that, with the expanded range of silicone hydrogel lenses on the market, including the introduction of custom-made lenses (sifilcon A, CIBA VISION), several options exist for most contact lens wearers. The challenge of prescribing the most suitable lens and solution for each patient based on their contact lens history, eye shape and prescription, and lifestyle, makes for interesting and rewarding practice.
References
- Tighe BJ. Silicone hydrogels: structure, properties and behaviour. In: Sweeney DF, ed.Silicone hydrogels: structure, properties and behaviour, Oxford, UK: Butterworth Heinemann, 2004; 1-27.
- Atkins N. Menicon PremiO: a new SiH for daily and extended wear. In.Menicon PremiO: a new SiH for daily and extended wear, 2007.
- Moezzi AM, Fonn D, Simpson TL. Overnight corneal swelling with silicone hydrogel contact lenses with high oxygen transmissibility. Eye Contact Lens 2006; 32: 277-80.
- Brennan NA, Coles ML, Connor HR, McIlroy RG. A 12-month prospective clinical trial of comfilcon A silicone-hydrogel contact lenses worn on a 30-day continuous wear basis. Cont Lens Anterior Eye 2007; 30: 108-18.
- O'Hare N, Stapleton F, Naduvilath T, Jalbert I, Sweeney DF, Holden BA. Interaction between the contact lens and the ocular surface in the etiology of superior epithelial arcuate lesions. Adv Exp Med Biol 2002; 506: 973-80.
- Dumbleton KA. Adverse events with silicone hydrogel continuous wear. Contact Lens Ant Eye 2002; 21: 318-24.
- Skotnitsky CC, Sweeney DF, Naduvilath TJ, Sankaridurg PR. The Incidence of Local and General Contact Lens Induced Papillary Conjunctivitis in Silicone Hydrogel Contact Lenses Invest Ophthalmol Vis Sci 2005.
- Willcox M, Naduvilath T, Carnt N, Zhao Z. Protein and lipid deposits on lenses are affected by lens material and solution, and are associated with clinical performance. In.Protein and lipid deposits on lenses are affected by lens material and solution, and are associated with clinical performance, Italy, 2007.
- Andrasko G. Andrasko Corneal Staining Grid. In.Andrasko Corneal Staining Grid, 2006-2007.
- Tilia D, Jalbert I, Carnt N, Keay L, Naduvilath T, Willcox MDP, Papas E, Holden BA. Evaluation of solution toxicity associated with lens care products during silicone hydrogel lens wear AAO E-Abstract 060094 2006.
- Carnt N, Jalbert I, Stretton S, Naduvilath T, Papas E. Solution toxicity in soft contact lens daily wear is associated with corneal inflammation. Optom Vis Sci 2007; 84: 309-15.
- Papas E, Dahms A, Tahhan N, Carnt N. Power profiles and short term visual performance of soft contact lenses. In.Power profiles and short term visual performance of soft contact lenses, Ft Lauderdale: Invest Ophthalmol Vis Sci, 2005.
|